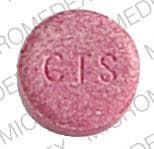
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Nexafed Sinus Pressure + Pain: Acetaminophen 325 mg and pseudoephedrine hydrochloride 30 mg
Pharmacology
Mechanism of Action
Acetaminophen: Although not fully elucidated, the analgesic effects are believed to be due to activation of descending serotonergic inhibitory pathways in the CNS. Interactions with other nociceptive systems may be involved as well (Smith 2009). Antipyresis is produced from inhibition of the hypothalamic heat-regulating center.
Pseudoephedrine: Causes vasoconstriction of the arterioles of the nasal mucosa.
Use: Labeled Indications
Sinus congestion/headache: Temporary relief of headache, sinus congestion and pressure, nasal congestion, and minor aches and pains
Contraindications
OTC labeling: When used for self-medication, do not use with any other drug containing acetaminophen; in combination with or within 14 days of stopping an MAOI.
Dosage and Administration
Dosing: Adult
Sinus congestion/headache: Oral: 2 tablets every 4 to 6 hours as needed (maximum: 8 tablets [acetaminophen 2,600 mg/pseudoephedrine 240 mg] per day).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Sinus congestion/headache: Oral: Children ≥12 years of age and Adolescents: Refer to adult dosing.
Storage
Store at 15°C to 30°C (59°F to 86°F).
Acetaminophen and Pseudoephedrine Images
Drug Interactions
Alcohol (Ethyl): May enhance the hepatotoxic effect of Acetaminophen. Monitor therapy
Alkalinizing Agents: May increase the serum concentration of Alpha-/Beta-Agonists (Indirect-Acting). Monitor therapy
Alpha1-Blockers: May diminish the vasoconstricting effect of Alpha-/Beta-Agonists. Similarly, Alpha-/Beta-Agonists may antagonize Alpha1-Blocker vasodilation. Monitor therapy
AtoMOXetine: May enhance the hypertensive effect of Sympathomimetics. AtoMOXetine may enhance the tachycardic effect of Sympathomimetics. Monitor therapy
Barbiturates: May increase the metabolism of Acetaminophen. This may 1) diminish the effect of acetaminophen; and 2) increase the risk of liver damage. Exceptions: Amobarbital; Butabarbital; Butalbital; Methohexital; PENTobarbital; Secobarbital; Thiopental. Monitor therapy
Benzylpenicilloyl Polylysine: Alpha-/Beta-Agonists may diminish the diagnostic effect of Benzylpenicilloyl Polylysine. Management: Consider use of a histamine skin test as a positive control to assess a patient's ability to mount a wheal and flare response. Consider therapy modification
Busulfan: Acetaminophen may increase the serum concentration of Busulfan. Monitor therapy
Cannabinoid-Containing Products: May enhance the tachycardic effect of Sympathomimetics. Exceptions: Cannabidiol. Monitor therapy
CarBAMazepine: May increase the metabolism of Acetaminophen. This may 1) diminish the effect of acetaminophen; and 2) increase the risk of liver damage. Monitor therapy
Carbonic Anhydrase Inhibitors: May increase the serum concentration of Alpha-/Beta-Agonists (Indirect-Acting). Monitor therapy
Chloroprocaine: May enhance the hypertensive effect of Alpha-/Beta-Agonists. Monitor therapy
Cocaine (Topical): May enhance the hypertensive effect of Sympathomimetics. Management: Consider alternatives to use of this combination when possible. Monitor closely for substantially increased blood pressure or heart rate and for any evidence of myocardial ischemia with concurrent use. Consider therapy modification
Dapsone (Topical): May enhance the adverse/toxic effect of Methemoglobinemia Associated Agents. Monitor therapy
Dasatinib: Acetaminophen may enhance the hepatotoxic effect of Dasatinib. Dasatinib may increase the serum concentration of Acetaminophen. Consider therapy modification
Doxofylline: Sympathomimetics may enhance the adverse/toxic effect of Doxofylline. Monitor therapy
Ergot Derivatives: May enhance the hypertensive effect of Alpha-/Beta-Agonists. Ergot Derivatives may enhance the vasoconstricting effect of Alpha-/Beta-Agonists. Exceptions: Ergoloid Mesylates; Nicergoline. Avoid combination
FentaNYL: Alpha-/Beta-Agonists (Indirect-Acting) may decrease the serum concentration of FentaNYL. Specifically, fentanyl nasal spray serum concentrations may decrease and onset of effect may be delayed. Monitor therapy
Flucloxacillin: May enhance the adverse/toxic effect of Acetaminophen. Specifically, the risk for high anion gap metabolic acidosis may be increased. Monitor therapy
Fosphenytoin-Phenytoin: May decrease the serum concentration of Acetaminophen. Specifically, serum concentrations of acetaminophen may be decreased (leading to decreased efficacy), but the formation of the toxic N-acetyl-p-benzoquinone imine (NAPQI) metabolite may be increased (leading to increased hepatotoxicity). Monitor therapy
Guanethidine: May enhance the arrhythmogenic effect of Sympathomimetics. Guanethidine may enhance the hypertensive effect of Sympathomimetics. Monitor therapy
Imatinib: Acetaminophen may enhance the hepatotoxic effect of Imatinib. Monitor therapy
Iobenguane Radiopharmaceutical Products: Alpha-/Beta-Agonists (Indirect-Acting) may diminish the therapeutic effect of Iobenguane Radiopharmaceutical Products. Management: Discontinue all drugs that may inhibit or interfere with catecholamine transport or uptake for at least 5 biological half-lives before iobenguane administration. Do not administer these drugs until at least 7 days after each iobenguane dose. Avoid combination
Isoniazid: May enhance the adverse/toxic effect of Acetaminophen. Monitor therapy
LamoTRIgine: Acetaminophen may decrease the serum concentration of LamoTRIgine. Monitor therapy
Linezolid: May enhance the hypertensive effect of Sympathomimetics. Management: Reduce initial doses of sympathomimetic agents, and closely monitor for enhanced pressor response, in patients receiving linezolid. Specific dose adjustment recommendations are not presently available. Consider therapy modification
Local Anesthetics: Methemoglobinemia Associated Agents may enhance the adverse/toxic effect of Local Anesthetics. Specifically, the risk for methemoglobinemia may be increased. Monitor therapy
MetyraPONE: May increase the serum concentration of Acetaminophen. More importantly, by inhibiting the conjugative metabolism of acetaminophen, metyrapone may shift the metabolism towards the oxidative route that produces a hepatotoxic metabolite. Monitor therapy
Mipomersen: Acetaminophen may enhance the hepatotoxic effect of Mipomersen. Monitor therapy
Monoamine Oxidase Inhibitors: May enhance the hypertensive effect of Alpha-/Beta-Agonists (Indirect-Acting). While linezolid is expected to interact via this mechanism, management recommendations differ from other monoamine oxidase inhibitors. Refer to linezolid specific monographs for details. Exceptions: Linezolid. Avoid combination
Nitric Oxide: May enhance the adverse/toxic effect of Methemoglobinemia Associated Agents. Combinations of these agents may increase the likelihood of significant methemoglobinemia. Monitor therapy
Phenylephrine (Systemic): Acetaminophen may increase the serum concentration of Phenylephrine (Systemic). Monitor therapy
Prilocaine: Methemoglobinemia Associated Agents may enhance the adverse/toxic effect of Prilocaine. Combinations of these agents may increase the likelihood of significant methemoglobinemia. Management: Monitor patients for signs of methemoglobinemia (e.g., hypoxia, cyanosis) when prilocaine is used in combination with other agents associated with development of methemoglobinemia. Avoid lidocaine/prilocaine in infants receiving such agents. Monitor therapy
Probenecid: May increase the serum concentration of Acetaminophen. Probenecid may also limit the formation of at least one major non-toxic metabolite, possibly increasing the potential for formation of the toxic NAPQI metabolite. Consider therapy modification
Serotonin/Norepinephrine Reuptake Inhibitors: May enhance the tachycardic effect of Alpha-/Beta-Agonists. Serotonin/Norepinephrine Reuptake Inhibitors may enhance the vasopressor effect of Alpha-/Beta-Agonists. Consider therapy modification
Sodium Nitrite: Methemoglobinemia Associated Agents may enhance the adverse/toxic effect of Sodium Nitrite. Combinations of these agents may increase the likelihood of significant methemoglobinemia. Monitor therapy
Solriamfetol: Sympathomimetics may enhance the hypertensive effect of Solriamfetol. Monitor therapy
SORAfenib: Acetaminophen may enhance the hepatotoxic effect of SORAfenib. SORAfenib may increase the serum concentration of Acetaminophen. Consider therapy modification
Spironolactone: May diminish the vasoconstricting effect of Alpha-/Beta-Agonists. Monitor therapy
Sympathomimetics: May enhance the adverse/toxic effect of other Sympathomimetics. Monitor therapy
Tedizolid: May enhance the hypertensive effect of Sympathomimetics. Tedizolid may enhance the tachycardic effect of Sympathomimetics. Monitor therapy
Tricyclic Antidepressants: May enhance the vasopressor effect of Alpha-/Beta-Agonists. Management: Avoid, if possible, the use of alpha-/beta-agonists in patients receiving tricyclic antidepressants. If combined, monitor for evidence of increased pressor effects and consider reductions in initial dosages of the alpha-/beta-agonist. Consider therapy modification
Urinary Acidifying Agents: May decrease the serum concentration of Alpha-/Beta-Agonists (Indirect-Acting). Monitor therapy
Vitamin K Antagonists (eg, warfarin): Acetaminophen may enhance the anticoagulant effect of Vitamin K Antagonists. This appears most likely with daily acetaminophen doses exceeding 1.3 or 2 g/day for multiple consecutive days. Monitor therapy
Test Interactions
See individual agents.
Adverse Reactions
See individual agents.
Warnings/Precautions
Concerns related to adverse effects:
- Hepatotoxicity: Acetaminophen has been associated with acute liver failure, at times resulting in liver transplant and death. Hepatotoxicity is usually associated with excessive acetaminophen intake and often involves more than one product that contains acetaminophen. Do not exceed the maximum recommended daily dose (>4 g daily in adults). In addition, long-term daily dosing may also result in liver damage in some patients.
- Hypersensitivity/anaphylactic reactions: Hypersensitivity and anaphylactic reactions have been reported; discontinue immediately if symptoms of allergic or hypersensitivity reactions occur.
- Skin reactions: Rarely, acetaminophen may cause serious and potentially fatal skin reactions such as acute generalized exanthematous pustulosis, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). Discontinue treatment if severe skin reactions develop.
Disease-related concerns:
- Cardiovascular disease: Use with caution in patients with cardiovascular disease (including hypertension and ischemic heart disease).
- Diabetes: Use with caution in patients with diabetes mellitus.
- G6PD deficiency: Use with caution in patients with known G6PD deficiency.
- Hepatic impairment: Use caution in patients with hepatic impairment or active liver disease. Use with caution in patients with alcoholic liver disease; consuming ≥3 alcoholic drinks/day may increase the risk of liver damage. Avoid ethanol or limit to <3 drinks/day.
- Increased intraocular pressure/glaucoma: Use with caution in patients with increased intraocular pressure or angle-closure glaucoma.
- Prostatic hyperplasia/urinary obstruction: Use with caution in patients with prostatic hyperplasia and/or GU obstruction.
- Thyroid dysfunction: Use with caution in patients with thyroid dysfunction.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Elderly: Use with caution in the elderly; may be more sensitive to adverse effects.
Other warnings/precautions:
- Dosage limit: Limit acetaminophen dose to <4 g/day (adults) or <2.6 g/day (children <12 years of age).
- Self-medication (OTC use): When used for self-medication (OTC), discontinue use and notify health care provider if pain or nasal congestion worsens or lasts >7 days; fever worsens or lasts >3 days; if any new symptoms or nervousness, dizziness, or sleeplessness occur; or if redness or swelling is present.
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience dizziness, anxiety, or trouble sleeping. Have patient report immediately to prescriber signs of hepatic impairment, unable to pass urine, oliguria, or signs of Stevens-Johnson syndrome/toxic epidermal necrolysis (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for healthcare professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience and judgment in diagnosing, treating and advising patients.