Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral, as hydrochloride:
Generic: 10 mg, 20 mg
Pharmacology
Mechanism of Action
Competitively blocks beta1-receptors, with little or no effect on beta2-receptors
Pharmacokinetics/Pharmacodynamics
Absorption
~100%
Metabolism
Hepatic to multiple metabolites
Excretion
Urine (>80%, as unchanged drug [15%] and inactive metabolites)
Onset of Action
1 to 1.5 hours
Time to Peak
1.5 to 6 hours
Half-Life Elimination
14 to 22 hours; prolonged in hepatic disease and/or chronic renal failure. In patients with chronic renal failure undergoing dialysis, the half-life and AUC are approximately doubled.
Protein Binding
~50%
Use in Specific Populations
Special Populations: Elderly
Elimination may be decreased.
Use: Labeled Indications
Hypertension: Management of hypertension. Note: Beta-blockers are not recommended as first-line therapy (ACC/AHA [Whelton 2017]).
Use: Off Label
Acute MIyes
According to the American College of Cardiology Foundation/American Heart Association (ACC/AHA) guidelines for the management of ST-elevation myocardial infarction (STEMI) and the ACC/AHA guidelines for the management of non-ST-elevation ACS (NSTE-ACS), oral beta-blockers should be initiated within the first 24 hours unless the patient has signs of heart failure, evidence of a low-output state, an increased risk for cardiogenic shock, or other contraindications. However, recommendations do not specify any particular beta-blocking agent for optimal treatment of NSTE-ACS. Thus, clinicians must use practical experience to determine proper therapy in managing patients ACC/AHA [Amsterdam 2014], ACC/AHA [O'Gara 2013].
Atrial fibrillation (rate control)byes
Data from a prospective randomized crossover study using the combination of low-dose betaxolol with digoxin compared to the use of low-dose diltiazem and digoxin supports the use of betaxolol as an effective agent for rate control in patients with atrial fibrillation Koh 1995. Additional trials may be necessary to further define the role of betaxolol in the treatment of this condition.
Oral beta-blocker use is recommended as a rate control option by the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guidelines for the management of patients with atrial fibrillation. However, betaxolol is not one of the more commonly administered or studied beta-blockers for this indication.
Chronic stable anginabyes
Data from a placebo-controlled trial demonstrated that betaxolol, when added to nifepidine or diltiazem, can reduce the onset of exercise-induced angina in those with stable angina. The addition of betaxolol to calcium channel blocker warrants monitoring of blood pressure and heart rate; syncope can occur with this combination of therapy Glasser 1994. Data from a randomized controlled trial which compared betaxolol to metoprolol tartrate in those with stable angina also supports the use of betaxolol as an effective antianginal Kardas 2007. Additional trials may be necessary to further define the role of betaxolol in the treatment of chronic stable angina.
Based on the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons guideline for the management of stable ischemic heart disease, beta-blocker therapy is effective in controlling anginal symptoms; efficacy appears equal among agents in this class.
Postoperative atrial fibrillation associated with cardiac surgery (prevention)byes
Data from an open-label randomized multicenter trial comparing once daily betaxolol to twice daily metoprolol tartrate administered 2 days before and after coronary artery bypass graft surgery to prevent postoperative atrial fibrillation supports the use of betaxolol as an effective agent to reduce the risk of rate of early postoperative atrial fibrillation Iliuta 2009. Additional trials may be necessary to further define the role of betaxolol in the prevention of atrial fibrillation following cardiac surgery.
Based on the American College of Cardiology Foundation/American Heart Association guideline for coronary artery bypass graft surgery beta-blockers should be administered at least 24 hours prior to bypass surgery and reinitiated after surgery, unless contraindicated, to reduce the risk and consequences of atrial fibrillation.
Contraindications
Hypersensitivity to betaxolol or any component of the formulation; sinus bradycardia; heart block greater than first-degree (except in patients with a functioning artificial pacemaker); cardiogenic shock; uncompensated cardiac failure
Documentation of allergenic cross-reactivity for beta-blockers is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Dosage and Administration
Dosing: Adult
Atrial fibrillation (rate control) (off-label use): 20 mg once daily; may use in combination with digoxin (Koh 1995)
Chronic stable angina (off-label use): 20 mg once daily (Glasser 1994)
Hypertension (alternative agent): Oral: Initial: 5 to 10 mg once daily; titrate as needed after 7 to 14 days based on patient response up to 20 mg once daily (ACC/AHA [Whelton 2017]). Note: Increasing the dose beyond 20 mg once daily has not been shown to produce further antihypertensive effect.
Postoperative atrial fibrillation associated with cardiac surgery (prevention) (off-label use): 20 mg once daily (Iliuta 2009)
Dosing: Geriatric
Refer to adult dosing.
Hypertension: Oral: Initial: 5 mg once daily
Administration
Oral: Absorption is not affected by food.
Storage
Store at 15°C to 25°C (59°F to 77°F). Avoid freezing.
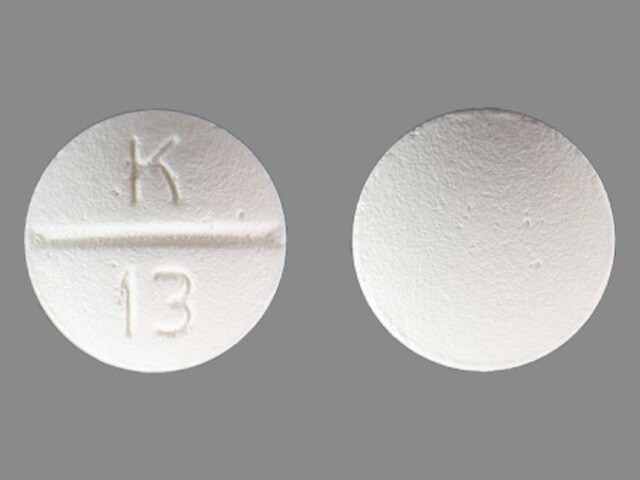
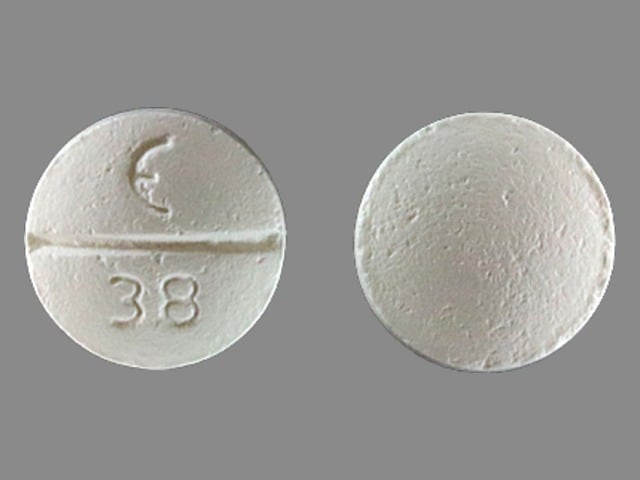
Betaxolol (Systemic) Images
Drug Interactions
Acetylcholinesterase Inhibitors: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Alpha1-Blockers: Beta-Blockers may enhance the orthostatic hypotensive effect of Alpha1-Blockers. The risk associated with ophthalmic products is probably less than systemic products. Monitor therapy
Alpha2-Agonists: May enhance the AV-blocking effect of Beta-Blockers. Sinus node dysfunction may also be enhanced. Beta-Blockers may enhance the rebound hypertensive effect of Alpha2-Agonists. This effect can occur when the Alpha2-Agonist is abruptly withdrawn. Management: Closely monitor heart rate during treatment with a beta blocker and clonidine. Withdraw beta blockers several days before clonidine withdrawal when possible, and monitor blood pressure closely. Recommendations for other alpha2-agonists are unavailable. Exceptions: Apraclonidine. Consider therapy modification
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Aminoquinolines (Antimalarial): May decrease the metabolism of Beta-Blockers. Monitor therapy
Amiodarone: May enhance the bradycardic effect of Beta-Blockers. Possibly to the point of cardiac arrest. Amiodarone may increase the serum concentration of Beta-Blockers. Monitor therapy
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Antipsychotic Agents (Phenothiazines): May enhance the hypotensive effect of Beta-Blockers. Beta-Blockers may decrease the metabolism of Antipsychotic Agents (Phenothiazines). Antipsychotic Agents (Phenothiazines) may decrease the metabolism of Beta-Blockers. Monitor therapy
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Barbiturates: May decrease the serum concentration of Beta-Blockers. Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Beta2-Agonists: Beta-Blockers (Beta1 Selective) may diminish the bronchodilatory effect of Beta2-Agonists. Of particular concern with nonselective beta-blockers or higher doses of the beta1 selective beta-blockers. Monitor therapy
Bradycardia-Causing Agents: May enhance the bradycardic effect of other Bradycardia-Causing Agents. Monitor therapy
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Bupivacaine: Beta-Blockers may increase the serum concentration of Bupivacaine. Monitor therapy
Calcium Channel Blockers (Nondihydropyridine): May enhance the hypotensive effect of Beta-Blockers. Bradycardia and signs of heart failure have also been reported. Calcium Channel Blockers (Nondihydropyridine) may increase the serum concentration of Beta-Blockers. Exceptions: Bepridil. Monitor therapy
Cardiac Glycosides: Beta-Blockers may enhance the bradycardic effect of Cardiac Glycosides. Monitor therapy
Ceritinib: Bradycardia-Causing Agents may enhance the bradycardic effect of Ceritinib. Management: If this combination cannot be avoided, monitor patients for evidence of symptomatic bradycardia, and closely monitor blood pressure and heart rate during therapy. Exceptions are discussed in separate monographs. Consider therapy modification
Cholinergic Agonists: Beta-Blockers may enhance the adverse/toxic effect of Cholinergic Agonists. Of particular concern are the potential for cardiac conduction abnormalities and bronchoconstriction. Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Dipyridamole: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Disopyramide: May enhance the bradycardic effect of Beta-Blockers. Beta-Blockers may enhance the negative inotropic effect of Disopyramide. Monitor therapy
Dronedarone: May enhance the bradycardic effect of Beta-Blockers. Dronedarone may increase the serum concentration of Beta-Blockers. This likely applies only to those agents that are metabolized by CYP2D6. Management: Use lower initial beta-blocker doses; adequate tolerance of the combination, based on ECG findings, should be confirmed prior to any increase in beta-blocker dose. Consider therapy modification
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
EPINEPHrine (Nasal): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Nasal). Monitor therapy
EPINEPHrine (Oral Inhalation): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Oral Inhalation). Monitor therapy
Epinephrine (Racemic): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of Epinephrine (Racemic). Monitor therapy
EPINEPHrine (Systemic): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Systemic). Monitor therapy
Ergot Derivatives: Beta-Blockers may enhance the vasoconstricting effect of Ergot Derivatives. Exceptions: Nicergoline. Consider therapy modification
Fexinidazole [INT]: Bradycardia-Causing Agents may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Fingolimod: Beta-Blockers may enhance the bradycardic effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and beta-blockers if possible. If coadministration is necessary, patients should have overnight continuous ECG monitoring conducted after the first dose of fingolimod. Monitor patients for bradycardia. Consider therapy modification
Floctafenine: May enhance the adverse/toxic effect of Beta-Blockers. Avoid combination
Grass Pollen Allergen Extract (5 Grass Extract): Beta-Blockers may enhance the adverse/toxic effect of Grass Pollen Allergen Extract (5 Grass Extract). More specifically, Beta-Blockers may inhibit the ability to effectively treat severe allergic reactions to Grass Pollen Allergen Extract (5 Grass Extract) with epinephrine. Some other effects of epinephrine may be unaffected or even enhanced (e.g., vasoconstriction) during treatment with Beta-Blockers. Consider therapy modification
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Insulins: Beta-Blockers may enhance the hypoglycemic effect of Insulins. Monitor therapy
Ivabradine: Bradycardia-Causing Agents may enhance the bradycardic effect of Ivabradine. Monitor therapy
Lacosamide: Bradycardia-Causing Agents may enhance the AV-blocking effect of Lacosamide. Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Lidocaine (Systemic): Beta-Blockers may increase the serum concentration of Lidocaine (Systemic). Monitor therapy
Lidocaine (Topical): Beta-Blockers may increase the serum concentration of Lidocaine (Topical). Monitor therapy
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Mepivacaine: Beta-Blockers may increase the serum concentration of Mepivacaine. Monitor therapy
Methacholine: Beta-Blockers may enhance the adverse/toxic effect of Methacholine. Monitor therapy
Methoxyflurane: May enhance the hypotensive effect of Beta-Blockers. Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Midodrine: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
NIFEdipine: May enhance the hypotensive effect of Beta-Blockers. NIFEdipine may enhance the negative inotropic effect of Beta-Blockers. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: May diminish the antihypertensive effect of Beta-Blockers. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Opioids (Anilidopiperidine): May enhance the bradycardic effect of Beta-Blockers. Opioids (Anilidopiperidine) may enhance the hypotensive effect of Beta-Blockers. Monitor therapy
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Propafenone: May increase the serum concentration of Beta-Blockers. Propafenone possesses some independent beta blocking activity. Monitor therapy
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Regorafenib: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Reserpine: May enhance the hypotensive effect of Beta-Blockers. Monitor therapy
Rifamycin Derivatives: May decrease the serum concentration of Beta-Blockers. Exceptions: Rifabutin. Monitor therapy
Rivastigmine: May enhance the bradycardic effect of Beta-Blockers. Avoid combination
Ruxolitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Management: Ruxolitinib Canadian product labeling recommends avoiding use with bradycardia-causing agents to the extent possible. Monitor therapy
Siponimod: Bradycardia-Causing Agents may enhance the bradycardic effect of Siponimod. Management: Avoid coadministration of siponimod with drugs that may cause bradycardia. Consider therapy modification
Sulfonylureas: Beta-Blockers may enhance the hypoglycemic effect of Sulfonylureas. Cardioselective beta-blockers (eg, acebutolol, atenolol, metoprolol, and penbutolol) may be safer than nonselective beta-blockers. All beta-blockers appear to mask tachycardia as an initial symptom of hypoglycemia. Ophthalmic beta-blockers are probably associated with lower risk than systemic agents. Monitor therapy
Terlipressin: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Theophylline Derivatives: Beta-Blockers (Beta1 Selective) may diminish the bronchodilatory effect of Theophylline Derivatives. Management: Monitor for reduced theophylline efficacy during concomitant use with any beta-blocker. Beta-1 selective agents are less likely to antagonize theophylline than nonselective agents, but selectivity may be lost at higher doses. Monitor therapy
Tofacitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
Oral betaxolol may interfere with glaucoma screening tests; may lead to false-positive aldosterone/renin ratio (ARR) (Funder 2016)
Adverse Reactions
2% to 10%:
Cardiovascular: Bradycardia (6% to 8%; symptomatic bradycardia: ≤2%; dose-dependent), chest pain (2% to 7%), cold extremities (2%), palpitations (2%), edema (≤2%; similar to placebo)
Central nervous system: Fatigue (3% to 10%), insomnia (1% to 5%), lethargy (3%), paresthesia (2%)
Gastrointestinal: Nausea (2% to 6%), dyspepsia (4% to 5%), diarrhea (2%)
Hematologic & oncologic: Positive ANA titer (5%)
Neuromuscular & skeletal: Arthralgia (3% to 5%)
Respiratory: Dyspnea (2%), pharyngitis (2%)
<2%, postmarketing, and/or case reports: Abnormal dreams, abnormality in thinking, acidosis, alopecia, amnesia, anemia, angina pectoris, anorexia, arthropathy, ataxia, atrioventricular block, blepharitis, breast fibroadenosis, bronchitis, bronchospasm, cardiac arrhythmia, cardiac failure, cataract, cerebrovascular disease, conjunctivitis, constipation, cough, cystitis, deafness, decreased libido, depression, diabetes mellitus, diaphoresis, dysgeusia, dysphagia, dysuria, emotional lability, epistaxis, erythematous rash, exacerbation of psoriasis, fever, flushing, hallucination, hemophthalmos, hypercholesterolemia, hyperglycemia, hyperkalemia, hyperlipidemia, hypersensitivity reaction, hypertension, hypertrichosis, hyperuricemia, hypoglycemia, hypokalemia, hypotension, impotence, increased lactate dehydrogenase, increased serum ALT, increased serum AST, influenza, intermittent claudication, iritis, labyrinth disease, leukocytosis, lymphadenopathy, malaise, menstrual disease, muscle cramps, myocardial infarction, nervousness, neuralgia, neuropathy, numbness, oliguria, peripheral ischemia, Peyronie’s disease, pneumonia, prostatitis, proteinuria, pruritus, purpura, renal insufficiency, rhinitis, rigors, scotoma, sinusitis, skin rash, stupor, syncope, tendonitis, thrombocytopenia, thrombophlebitis, thrombosis, tinnitus, tremor, twitching, visual disturbance, vomiting, weight gain, weight loss, xerostomia
Warnings/Precautions
Concerns related to adverse events:
- Anaphylactic reactions: Use caution with history of severe anaphylaxis to allergens; patients taking beta-blockers may become more sensitive to repeated challenges. Treatment of anaphylaxis (eg, epinephrine) in patients taking beta-blockers may be ineffective or promote undesirable effects.
Disease-related concerns:
- Bronchospastic disease: In general, patients with bronchospastic disease should not receive beta-blockers; however, betaxolol, with B1 selectivity, may be used cautiously with the lowest possible dose (eg, 5 to 10 mg/day), availability of a bronchodilator, and close monitoring; if a dosage increase is indicated, administer in divided doses.
- Cerebrovascular insufficiency: Use with caution in patients with cerebrovascular insufficiency; hypotension and decreased heart rate may reduce cerebral blood flow.
- Conduction abnormality: Consider preexisting conditions such as sick sinus syndrome before initiating therapy.
- Diabetes: Use with caution in patients with diabetes mellitus; may potentiate and/or mask signs and symptoms of hypoglycemia.
- Heart failure (HF): Use with caution in patients with compensated heart failure and monitor for a worsening of the condition. There is limited data evaluating the efficacy of betaxolol in HF; use is not recommended (Figulla HR 2006; Yancy 2013).
- Myasthenia gravis: Use with caution in patients with myasthenia gravis; may potentiate myasthenia-related muscle weakness, including diplopia and ptosis.
- Peripheral vascular disease (PVD) and Raynaud disease: May precipitate or aggravate symptoms of arterial insufficiency in patients with PVD and Raynaud disease. Use with caution and monitor for progression of arterial obstruction.
- Pheochromocytoma (untreated): Adequate alpha-blockade is required prior to use of any beta-blocker.
- Prinzmetal variant angina: Beta-blockers without alpha1-adrenergic receptor blocking activity should be avoided in patients with Prinzmetal variant angina since unopposed alpha1-adrenergic receptors mediate coronary vasoconstriction and can worsen anginal symptoms (Mayer 1998).
- Psoriasis: Beta-blocker use has been associated with induction or exacerbation of psoriasis but cause and effect has not been firmly established.
- Renal impairment: Use with caution in patients with renal impairment; dosage adjustment required in severe impairment and in patients on dialysis.
- Thyroid disease: May mask signs of hyperthyroidism (eg, tachycardia). If hyperthyroidism is suspected, carefully manage and monitor; abrupt withdrawal may precipitate thyroid storm.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Elderly: Bradycardia may be observed more frequently in elderly patients (>65 years of age); dosage reductions may be necessary.
Other warnings/precautions:
- Abrupt withdrawal: Beta-blocker therapy should not be withdrawn abruptly (particularly in patients with CAD), but gradually tapered to avoid acute tachycardia, hypertension, ischemia, and/or angina exacerbation. Severe exacerbation of angina, ventricular arrhythmias, and MI have been reported following abrupt withdrawal of beta-blocker therapy. Temporary but prompt resumption of beta-blocker therapy may be indicated with worsening of angina or acute coronary insufficiency.
- Major surgery: Chronic beta-blocker therapy should not be routinely withdrawn prior to major surgery.
Monitoring Parameters
Blood pressure, pulse; baseline renal function
Hypertension: The 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults (ACC/AHA [Whelton 2017]):
Confirmed hypertension and known CVD or 10-year ASCVD risk ≥10%: Target blood pressure <130/80 mm Hg is recommended.
Confirmed hypertension without markers of increased ASCVD risk: Target blood pressure <130/80 mm Hg may be reasonable.
Pregnancy
Pregnancy Risk Factor
C
Pregnancy Considerations
Betaxolol crosses the placenta (Morselli 1990).
Following maternal use of betaxolol, the beta-blocker effects may persist in the neonate for several days after birth. The risk of cardiac and pulmonary complications is increased in the neonate. Bradycardia, hypoglycemia, and respiratory distress have been reported and monitoring of the neonate for 3 to 5 days after birth is recommended.
Chronic maternal hypertension is also associated with adverse events in the fetus/infant. Chronic maternal hypertension may increase the risk of birth defects, low birth weight, premature delivery, stillbirth, and neonatal death. Actual fetal/neonatal risks may be related to duration and severity of maternal hypertension. Untreated chronic hypertension may also increase the risks of adverse maternal outcomes, including gestational diabetes, preeclampsia, delivery complications, stroke, and myocardial infarction (ACOG 203 2019).
The maternal half-life and serum concentration of betaxolol immediately postpartum are not significantly different than what is observed in nonpregnant women (Boutroy 1990; Morselli 1990). When treatment of chronic hypertension in pregnancy is indicated, agents other than betaxolol are preferred (ACOG 203 2019; ESC [Regitz-Zagrosek 2018]; Magee 2014). Females with preexisting hypertension may continue their medication during pregnancy unless contraindications exist (ESC [Regitz-Zagrosek 2018]).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience joint pain, nausea, loss of strength and energy, headache, or trouble sleeping. Have patient report immediately to prescriber severe dizziness, passing out, shortness of breath, excessive weight gain, swelling of arms or legs, chest pain, slow heartbeat, or abnormal heartbeat (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.