What is Cotempla XR-ODT?
Cotempla XR-ODT is a central nervous system (CNS) stimulant prescription medicine used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children 6 to 17 years of age. Cotempla XR-ODT may help increase attention and decrease impulsiveness and hyperactivity in children 6 to 17 years of age with ADHD.
It is not known if Cotempla XR-ODT is safe and effective in children under 6 years of age.
Cotempla XR-ODT is a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep Cotempla XR-ODT in a safe place to protect it from theft. Never give your Cotempla XR-ODT to anyone else, because it may cause death or harm them. Selling or giving away Cotempla XR-ODT may harm others and is against the law.
What is the most important information I should know about Cotempla XR-ODT?
Cotempla XR-ODT can cause serious side effects, including:
- Abuse and dependence. Cotempla XR-ODT, other methylphenidate containing medicines, and amphetamines have a high chance for abuse and can cause physical and psychological dependence. Your healthcare provider should check your child for signs of abuse and dependence before and during treatment with Cotempla XR-ODT.
- Tell your healthcare provider if your child has ever abused or been dependent on alcohol, prescription medicines, or street drugs.
- Your healthcare provider can tell you more about the differences between physical and psychological dependence and drug addiction.
- Heart-related problems, including: Your healthcare provider should check your child carefully for heart problems before starting Cotempla XR-ODT. Tell your healthcare provider if your child has any heart problems, heart defects, high blood pressure, or a family history of these problems.
Your healthcare provider should check your child's blood pressure and heart rate regularly during treatment with Cotempla XR-ODT.
Call your healthcare provider or go to the nearest hospital emergency room right away if your child has any signs of heart problems such as chest pain, shortness of breath, or fainting during treatment with Cotempla XR-ODT.- sudden death in children who have heart problems or heart defects
- increased blood pressure and heart rate
- Mental (psychiatric) problems, including:
- new or worse behavior and thought problems
- new or worse bipolar illness
- new psychotic symptoms (such as hearing voices, or seeing or believing things that are not real) or new manic symptoms
Tell your healthcare provider about any mental problems your child has, or about a family history of suicide, bipolar illness, or depression.
Call your healthcare provider right away if your child has any new or worsening mental symptoms or problems during treatment with Cotempla XR-ODT, especially hearing voices, seeing or believing things that are not real, or new manic symptoms.
Who should not take Cotempla XR-ODT?
Do not give Cotempla XR-ODT to your child if they are:
- allergic to methylphenidate or any of the ingredients in Cotempla XR-ODT. See the end of this Medication Guide for a complete list of ingredients in Cotempla XR-ODT.
- taking, or has taken within the past 14 days, a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI).
What should I tell my healthcare provider before taking Cotempla XR-ODT?
Before taking Cotempla XR-ODT tell your child's healthcare provider about all medical conditions, including if your child:
- has heart problems, heart defects, or high blood pressure
- has mental problems including psychosis, mania, bipolar illness, or depression
- has circulation problems in fingers and toes
- is pregnant or plans to become pregnant. It is not known if Cotempla XR-ODT will harm the unborn baby.
- There is a pregnancy registry for females who are exposed to Cotempla XR-ODT during pregnancy. The purpose of the registry is to collect information about the health of females exposed to Cotempla XR-ODT and their baby. If your child becomes pregnant during treatment with Cotempla XR-ODT, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychostimulants. You can register by calling 1-866-961-2388.
- is breastfeeding or plans to breastfeed. Cotempla XR-ODT passes into breast milk. You and your healthcare provider should decide if your child will take Cotempla XR-ODT or breastfeed.
Tell your healthcare provider about all of the medicines that your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Cotempla XR-ODT and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted during treatment with Cotempla XR-ODT.
Your healthcare provider will decide whether Cotempla XR-ODT can be taken with other medicines. Especially tell your healthcare provider if your child takes:
- anti-depression medicines including MAOIs
Know the medicines that your child takes. Keep a list of the medicines with you to show your healthcare provider and pharmacist. Do not start any new medicine during treatment with Cotempla XR-ODT without talking to your healthcare provider first.
How should I take Cotempla XR-ODT?
- Take Cotempla XR-ODT exactly as prescribed by your healthcare provider.
- Your healthcare provider may change the dose if needed.
- Take Cotempla XR-ODT 1 time each day in the morning.
- Cotempla XR-ODT can be taken with or without food but take it the same way each time.
Take Cotempla XR-ODT as follows:
- Keep Cotempla XR-ODT in the blister pack until your child is ready to take it. Take Cotempla XR-ODT right after opening the blister pack. Do not store the tablet for future use.
- Use dry hands when opening the blister pack.
- Remove the tablet by peeling back the foil on the blister pack. Do not push the tablet through the foil.
- As soon as the blister is opened, remove the tablet and place it on the tongue. Do not chew or crush the tablet.
- The tablet will dissolve and can be swallowed with saliva. No liquid is needed to take the tablet.
Your healthcare provider may sometimes stop your child's Cotempla XR-ODT treatment for a while to check ADHD symptoms.
If your child takes too much Cotempla XR-ODT, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Cotempla XR-ODT?
You should avoid drinking alcohol during treatment with Cotempla XR-ODT.
What are the possible side effects of Cotempla XR-ODT?
Cotempla XR-ODT can cause serious side effects, including:
- See "What is the most important information I should know about Cotempla XR-ODT?"
- Painful and prolonged erections (priapism). Priapism has happened in males who take products that contain methylphenidate. If your child develops priapism, get medical help right away.
- Circulation problems in fingers and toes (peripheral vasculopathy, including Raynaud's phenomenon). Signs and symptoms may include:
- fingers or toes may feel numb, cool, painful
- fingers or toes may change color from pale, to blue, to red
- Tell your healthcare provider if your child has numbness, pain, skin color change, or sensitivity to temperature in their fingers or toes.
Call your healthcare provider right away if your child has any signs of unexplained wounds appearing on fingers or toes during treatment with Cotempla XR-ODT. - Slowing of growth (height and weight) in children. Children should have their height and weight checked often during treatment with Cotempla XR-ODT. Cotempla XR-ODT treatment may be stopped if your child is not gaining weight or height.
The most common side effects of methylphenidate products include:
- decreased appetite
- trouble sleeping
- nausea
- vomiting
- indigestion
- stomach pain
- weight loss
- anxiety
- dizziness
- irritability
- mood swings
- increased heart rate
- increased blood pressure
These are not all the possible side effects of Cotempla XR-ODT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
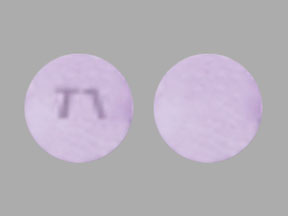
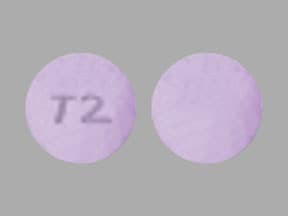
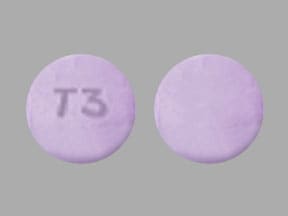
Cotempla XR-ODT Images
General information about the safe and effective use of Cotempla XR-ODT
Medicines are sometimes prescribed for purposes other than those listed in the Medication Guide. Do not use Cotempla XR-ODT for a condition for which it was not prescribed. Do not give Cotempla XR-ODT to other people, even if they have the same condition. It may harm them and it is against the law. You can ask your healthcare provider or pharmacist for information about Cotempla XR-ODT that was written for healthcare professionals.
How should I store Cotempla XR-ODT?
- Store Cotempla XR-ODT at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Cotempla XR-ODT in a safe place, like a locked cabinet.
- Store Cotempla XR-ODT in the blister packaging until it is ready to be taken.
- Dispose of remaining, unused, or expired Cotempla XR-ODT by a medicine take-back program at authorized collection sites such as retail pharmacies, hospital or clinic pharmacies, and law enforcement locations. If no take-back program or authorized collector is available, mix Cotempla XR-ODT with an undesirable, nontoxic substance such as dirt, cat litter, or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container such as a sealed plastic bag and throw away Cotempla XR-ODT in the household trash.
Keep Cotempla XR-ODT and all medicines out of the reach of children.
What are the ingredients in Cotempla XR-ODT?
Active Ingredient: Methylphenidate
Inactive Ingredients: Mannitol, Fructose, Microcrystalline Cellulose, Crospovidone, Methacrylic Acid, Polystyrene Sulfonate, Citric Acid, Colloidal Silicon Dioxide, Grape Flavor, Natural Masking Type Powder, Triethyl Citrate, Magnesium Stearate, Ethylcellulose, Sucralose, Lake Blend Purple, and Polyethylene Glycol