Boxed Warning
Hepatotoxicity including autoimmune hepatitis:
Daclizumab can cause severe liver injury, including autoimmune hepatitis and liver failure. Fatal cases have occurred. Liver injury, including autoimmune hepatitis and acute liver failure, can occur at any time during treatment with daclizumab, and with cases reported up to 5 months after the last dose of daclizumab.
Daclizumab is contraindicated in patients with preexisting hepatic disease or hepatic impairment. Prior to starting daclizumab, obtain serum transaminases (ALT and AST) and bilirubin levels.
Test transaminase levels and total bilirubin monthly and assess before the next dose of daclizumab. Follow transaminase levels and total bilirubin monthly for 6 months after the last dose of daclizumab. In case of elevation in transaminases or total bilirubin, treatment interruption or discontinuation may be required.
Other immune-mediated disorders:
In addition to autoimmune hepatitis, a variety of immune-mediated disorders including skin reactions, lymphadenopathy, noninfectious colitis, and other serious conditions can occur in patients treated with daclizumab. Overall, serious immune-mediated disorders were observed in 5% of patients treated with daclizumab. If a patient develops a serious immune-mediated disorder, consider stopping daclizumab and refer the patient to a specialist to ensure comprehensive diagnostic evaluation and appropriate treatment.
Systemic corticosteroids:
Some patients required systemic corticosteroids or other immunosuppressant treatment for autoimmune hepatitis or other immune-mediated disorders and continued this treatment after the last dose of daclizumab.
REMS Program:
Because of the risks of hepatic injury, including autoimmune hepatitis, and other immune-mediated disorders, daclizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ZINBRYTA REMS Program.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Solution Prefilled Syringe, Subcutaneous [preservative free]:
Zinbryta: 150 mg/mL (1 mL [DSC])
Pharmacology
Mechanism of Action
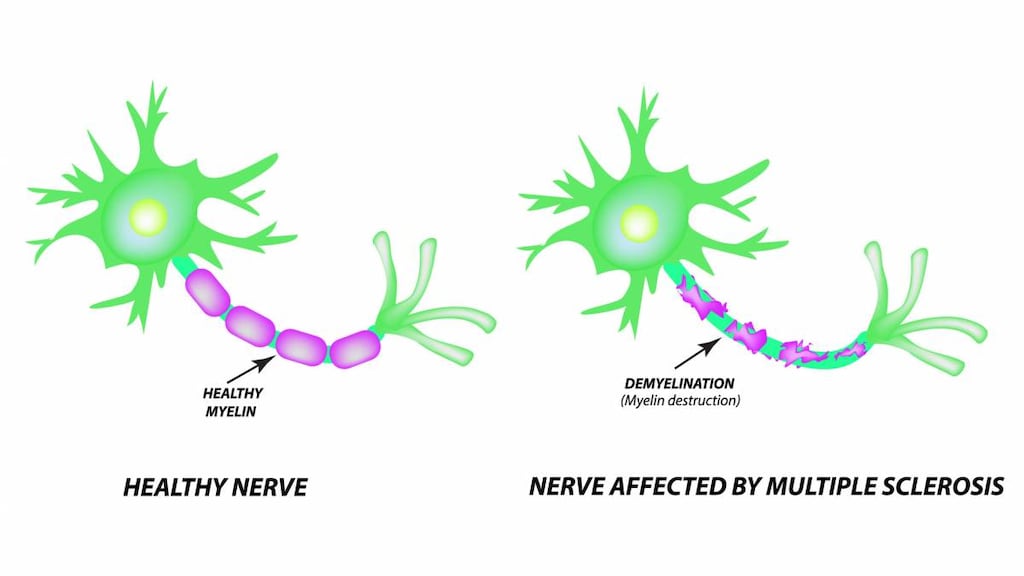
Daclizumab is a humanized monoclonal antibody which binds to the CD25 subunit of the high-affinity interleukin-2 (IL-2) receptor to prevent signaling at the high-affinity IL-2 receptor while allowing increased IL-2 availability for signaling at the intermediate-affinity IL-2 receptor (Gold 2013, Kappos 2015). Because IL-2 has a role in activating and regulating the immune system; CD25 antagonism may result in therapeutic benefit in multiple sclerosis (Gold 2013).
Pharmacokinetics/Pharmacodynamics
Distribution
Vd: SubQ: ~6.34 L
Metabolism
Catabolized to peptides and amino acids
Time to Peak
SubQ: 5 to 7 days
Half-Life Elimination
SubQ: 21 days
Use: Labeled Indications
Multiple sclerosis, relapsing: Treatment of relapsing forms of multiple sclerosis (MS) in adults. Daclizumab should generally be reserved for patients who have had an inadequate response to 2 or more medications indicated for the treatment of MS.
Contraindications
Hypersensitivity to daclizumab or any component of the formulation; preexisting hepatic disease or hepatic impairment, including ALT or AST at least 2 times the ULN; history of autoimmune hepatitis or other autoimmune condition involving the liver.
Dosage and Administration
Dosing: Adult
Note: Screen patients for hepatitis B and C prior to treatment initiation. Avoid initiating treatment in patients with severe active infection (including tuberculosis).
Multiple sclerosis, relapsing: SubQ: 150 mg once monthly (Gold 2013, Kappos 2015).
Missed dose: Administer a missed dose as soon as possible, but no more than 2 weeks late; after 2 weeks, skip the missed dose and administer the next dose on schedule. Administer only 1 dose at a time.
Dosing: Pediatric
Note: Screen patients for hepatitis B and C prior to treatment initiation. Avoid initiating treatment in patients with severe active infection (including tuberculosis).
Multiple sclerosis, relapsing: Adolescents ≥17 years: SubQ: 150 mg once monthly. Administer a missed dose as soon as possible, but no more than 2 weeks late; after 2 weeks, skip the missed dose and administer the next dose on schedule. Administer only 1 dose at a time.
Dosing adjustment for toxicity: Adolescents ≥17 years:
Anaphylaxis or other allergic reaction: Discontinue treatment; do not re-initiate.
Depression (severe) or suicidal ideation: Consider discontinuing treatment.
Dermatologic toxicity: Serious diffuse or inflammatory rashes: Discontinuation may be appropriate; consider referral to a dermatologist for evaluation prior to the next dose.
Immune-mediated disorders, serious: Consider discontinuing daclizumab and refer to a specialist; may require systemic corticosteroids or other immunosuppressant treatment.
Infection (serious): Consider withholding daclizumab until infection resolves.
Dosing: Adjustment for Toxicity
Anaphylaxis or other allergic reaction: Discontinue treatment; do not re-initiate.
Depression (severe) or suicidal ideation: Consider discontinuing treatment.
Dermatologic toxicity: Serious diffuse or inflammatory rashes: Discontinuation may be appropriate; consider referral to a dermatologist for evaluation prior to the next dose.
Immune-mediated disorders, serious: Consider discontinuing daclizumab and refer to a specialist; may require systemic corticosteroids or other immunosuppressant treatment.
Infection (serious): Consider withholding daclizumab until infection resolves.
Reconstitution
Allow to warm to room temperature by removing prefilled syringe from the refrigerator 30 minutes prior to injection. Do not use external heat sources (eg, hot water) to warm daclizumab. Do not place back into refrigerator after allowing product to warm to room temperature.
Administration
SubQ: For subcutaneous administration only. Administer subcutaneously into the thigh, abdomen, or back of the upper arm.
Remove prefilled syringe from refrigerator and allow to warm to room temperature 30 minutes prior to injection. Patients may be trained on proper technique for self-administration. Use each prefilled syringe one time and then place in a sharps container for disposal according to community guidelines.
Storage
Store at 2°C to 8°C (36°F to 46°F). Do not expose to temperatures above 30°C (86°F). Do not freeze (discard if frozen). Keep in the original carton to protect from light.
If refrigeration is unavailable, may be stored protected from light for up to 30 days at up to 30°C (86°F). Do not place back into the refrigerator after allowing it to warm to room temperature. Discard after 30 days without refrigeration.
Drug Interactions
Baricitinib: Immunosuppressants may enhance the immunosuppressive effect of Baricitinib. Management: Use of baricitinib in combination with potent immunosuppressants such as azathioprine or cyclosporine is not recommended. Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted. Consider therapy modification
BCG (Intravesical): Immunosuppressants may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Cladribine: May enhance the immunosuppressive effect of Immunosuppressants. Avoid combination
Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Monitor therapy
Denosumab: May enhance the adverse/toxic effect of Immunosuppressants. Specifically, the risk for serious infections may be increased. Monitor therapy
Echinacea: May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification
Fingolimod: Immunosuppressants may enhance the immunosuppressive effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and other immunosuppressants when possible. If combined, monitor patients closely for additive immunosuppressant effects (eg, infections). Consider therapy modification
Leflunomide: Immunosuppressants may enhance the adverse/toxic effect of Leflunomide. Specifically, the risk for hematologic toxicity such as pancytopenia, agranulocytosis, and/or thrombocytopenia may be increased. Management: Consider not using a leflunomide loading dose in patients receiving other immunosuppressants. Patients receiving both leflunomide and another immunosuppressant should be monitored for bone marrow suppression at least monthly. Consider therapy modification
Natalizumab: Immunosuppressants may enhance the adverse/toxic effect of Natalizumab. Specifically, the risk of concurrent infection may be increased. Avoid combination
Nivolumab: Immunosuppressants may diminish the therapeutic effect of Nivolumab. Consider therapy modification
Ocrelizumab: May enhance the immunosuppressive effect of Immunosuppressants. Monitor therapy
Pidotimod: Immunosuppressants may diminish the therapeutic effect of Pidotimod. Monitor therapy
Pimecrolimus: May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Roflumilast: May enhance the immunosuppressive effect of Immunosuppressants. Consider therapy modification
Siponimod: Immunosuppressants may enhance the immunosuppressive effect of Siponimod. Monitor therapy
Sipuleucel-T: Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Management: Evaluate patients to see if it is medically appropriate to reduce or discontinue therapy with immunosuppressants prior to initiating sipuleucel-T therapy. Consider therapy modification
Tacrolimus (Topical): May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide. Monitor therapy
Tofacitinib: Immunosuppressants may enhance the immunosuppressive effect of Tofacitinib. Management: Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted, and this warning seems particularly focused on more potent immunosuppressants. Consider therapy modification
Trastuzumab: May enhance the neutropenic effect of Immunosuppressants. Monitor therapy
Upadacitinib: Immunosuppressants may enhance the immunosuppressive effect of Upadacitinib. Avoid combination
Vaccines (Inactivated): Immunosuppressants may diminish the therapeutic effect of Vaccines (Inactivated). Management: Vaccine efficacy may be reduced. Complete all age-appropriate vaccinations at least 2 weeks prior to starting an immunosuppressant. If vaccinated during immunosuppressant therapy, revaccinate at least 3 months after immunosuppressant discontinuation. Consider therapy modification
Vaccines (Live): Daclizumab may enhance the adverse/toxic effect of Vaccines (Live). Daclizumab may diminish the therapeutic effect of Vaccines (Live). Avoid combination
Adverse Reactions
>10%:
Dermatologic: Allergic skin reaction (18% to 37%), skin rash (7% to 11%)
Immunologic: Autoimmune disease (13% to 32%)
Infection: Infection (50% to 65%)
Respiratory: Nasopharyngitis (25%), upper respiratory tract infection (9% to 17%)
1% to 10%:
Central nervous system: Depression (7% to 10%), seizure (1%)
Dermatologic: Dermatitis (3% to 9%), eczema (5%), acne vulgaris (3%)
Hematologic & oncologic: Lymphadenopathy (5%), anemia (3%)
Hepatic: Increased serum ALT (5% to 6%), increased serum AST (3% to 6%), hepatic injury (≤1%)
Infection: Influenza (9%)
Respiratory: Oropharyngeal pain (8%), bronchitis (7%), pharyngitis (6%), rhinitis (4%), tonsillitis (4%)
Miscellaneous: Fever (3%)
Frequency not defined:
Dermatologic: Desquamation, erythema, folliculitis, pruritus, psoriasis, skin photosensitivity, skin rash (toxic), xeroderma
Gastrointestinal: Diarrhea
Hematologic & oncologic: Decreased absolute lymphocyte count, lymphadenitis
Hepatic: Abnormal hepatic function tests, increased liver enzymes
Hypersensitivity: Hypersensitivity reaction (including anaphylaxis, angioedema, and urticaria)
Infection: Cytomegalovirus disease, viral infection
Respiratory: Laryngitis, pneumonia, respiratory tract infection
<1%, postmarketing, and/or case reports: Autoimmune hepatitis, colitis (serious; noninfectious), increased serum alkaline phosphatase (<2 x ULN), increased serum bilirubin (≥2 x ULN), increased serum transaminases (≥3 x ULN), malignant neoplasm of breast (more common in women), suicidal ideation
Warnings/Precautions
Concerns related to adverse effects:
- Hepatotoxicity: [US Boxed Warning]: Daclizumab may cause severe hepatic injury, including hepatic failure and autoimmune hepatitis; fatal cases have occurred. Hepatotoxicity, including autoimmune hepatitis, may occur at any time during daclizumab treatment; cases have been reported up to 5 months after the last dose. Daclizumab is contraindicated in patients with preexisting hepatic disease or hepatic impairment. Obtain serum transaminases (ALT and AST) and total bilirubin levels prior to treatment initiation, monthly before each dose, and monthly for 6 months after the last daclizumab dose. If transaminases or total bilirubin are elevated, treatment interruption or discontinuation may be required. Some patients required systemic corticosteroids or other immunosuppressant treatment for autoimmune hepatitis and continued this treatment after the last daclizumab dose. ALT or AST elevations (including rare elevations >20 times ULN) and bilirubin elevations have occurred during treatment and up to 4 months following the last dose. Monitor for clinical signs/symptoms of hepatic dysfunction (unexplained nausea, vomiting, abdominal pain, anorexia, fatigue, jaundice, and/or dark urine); if reported, promptly obtain serum transaminases and total bilirubin; may require treatment interruption or discontinuation. Prolonged transaminase elevations should be evaluated for alternate causes, including infection. If autoimmune hepatitis is suspected, promptly discontinue daclizumab; may require systemic corticosteroids and other immunosuppressant therapy. Long-term immunosuppression may be necessary. Other medications (including OTC medications, herbals, and dietary supplements) associated with hepatotoxicity should be used with caution in combination with daclizumab.
- Hypersensitivity: Anaphylaxis, angioedema, and urticaria may occur after the first dose or at any time during treatment. If anaphylaxis or other allergic reaction occurs, discontinue daclizumab and do not re-initiate.
- Immune-mediated disorders: [US Boxed Warning]: In addition to autoimmune hepatitis, other immune-mediated disorders such as skin reactions, lymphadenopathy, noninfectious colitis, and other serious conditions may occur with daclizumab. Serious immune-mediated conditions were observed in 5% of patients treated with daclizumab. If a serious immune-mediated disorder develops, consider stopping daclizumab and refer to a specialist for a comprehensive diagnostic evaluation and appropriate management. Some patients required systemic corticosteroids or other immunosuppressant treatment for immune-mediated disorders and continued this treatment after the last daclizumab dose. Concurrent or sequential immune-mediated disorders have been reported in some cases. Immune-mediated disorders have resulted in invasive diagnostic procedures, hospitalization, and/or prolonged systemic corticosteroids or other immunosuppressant therapy; some events had not resolved following daclizumab discontinuation and follow-up. Immune-mediated disorders have involved a single organ or systemic multi-organ inflammatory reactions. Monitor closely for immune-mediated disorders. Suspected immune-mediated disorders should be adequately evaluated to confirm etiology or to exclude other causes. Some immune-mediated disorders have required several months for resolution after the last dose and some had not resolved even several months after discontinuation.
- Colitis: Immune-mediated colitis, including colitis, ulcerative colitis, Crohn disease, microscopic colitis, inflammatory bowel disease, proctitis, and proctocolitis, has occurred. If symptoms of colitis (abdominal pain, fever, prolonged diarrhea) occur, consider discontinuing therapy and referring to a gastroenterologist.
- Dermatologic toxicity: Immune-mediated skin reactions, including rash, dermatitis, eczema, psoriatic conditions, and drug eruptions have occurred with daclizumab; erythema multiforme, erythema nodosum, and exfoliative rash have also been reported. Skin reactions may occur at any time during therapy. Photosensitivity has also been reported. Daclizumab may exacerbate eczema or psoriasis in patients with a history of those conditions. Dermatologic toxicity may be managed with topical or systemic corticosteroids, or immunosuppressant therapy (eg, tacrolimus). Some skin reactions have required discontinuation. Rash typically resolved at ~3 months, although some rashes remained unresolved at the final clinical evaluation. Infectious complications due to serious cutaneous reaction has been reported, including a rare fatality. Serious diffuse or inflammatory rashes should be evaluated by a dermatologist prior to the next dose; discontinuation may be appropriate.
- Hemolytic anemia: Immune-mediated hemolytic anemia has occurred; typically resolves with discontinuation of treatment, corticosteroid or other immunosuppressant treatment, and blood transfusions. If signs/symptoms of autoimmune hemolytic anemia occur, consider discontinuing treatment and referring to appropriate specialist for further evaluation and management.
- Lymphadenopathy: An increased incidence of lymphadenopathy was reported in patients who received daclizumab. The onset of lymphadenopathy or lymphadenitis may occur throughout the daclizumab treatment period. Serious events related to lymphadenopathy/lymphadenitis included infections, salivary neoplasm (benign), skin reactions, thrombocytopenia, and interstitial lung changes. Most cases resolved within 3 months, either with treatment continuing or discontinued. If lymph node biopsy is considered, the patient should undergo a full diagnostic exam by a specialist.
- Oral ulcers: Immune-mediated oral ulcers have been reported.
- Infection: Daclizumab increases the risk for infections (some serious). Upper respiratory tract infections, urinary tract infections, and viral infections were the most common infections; cytomegalovirus infections (hepatitis and pneumonia) have also occurred. In countries where tuberculosis is endemic, cases of tuberculosis occurred in patients receiving daclizumab. Evaluate patients at high risk for tuberculosis infection prior to treatment initiation. Avoid initiating daclizumab in patients with tuberculosis or other severe active infection. If serious infection develops while on therapy, consider withholding daclizumab until infection resolves. Screen for hepatitis B and C prior to treatment initiation.
- Neuropsychiatric events: Depression-related events occurred more frequently in patients receiving daclizumab than in patients who received placebo or comparator drug. Some events were serious, including suicidal ideation or attempt. Use daclizumab with caution in patients with prior or current depressive disorders. Patients or caregivers should immediately report symptoms of new or worsening depression or suicidal ideation. Consider discontinuing daclizumab if severe depression or suicidal ideation develops.
Disease related concerns:
- Hepatic impairment: Patients with signs/symptoms of hepatic impairment may be at increased risk for hepatotoxicity. Use is contraindicated in patients with preexisting hepatic disease, hepatic impairment, or history of autoimmune hepatitis (or other autoimmune condition involving the liver).
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Dosage form specific issues:
- Polysorbate 80: Some dosage forms may contain polysorbate 80 (also known as Tweens). Hypersensitivity reactions, usually a delayed reaction, have been reported following exposure to pharmaceutical products containing polysorbate 80 in certain individuals (Isaksson 2002, Lucente 2000, Shelley 1995). Thrombocytopenia, ascites, pulmonary deterioration, and renal and hepatic failure have been reported in premature neonates after receiving parenteral products containing polysorbate 80 (Alade 1986, CDC 1984). See manufacturer's labeling.
Other warnings/precautions:
- Immunizations: Immunization with live vaccines is not recommended during treatment and for up to 4 months after discontinuation. Consider necessary immunization prior to initiation of daclizumab treatment.
- Immunogenicity: Anti-daclizumab antibodies and neutralizing antibodies may develop, usually during the first year of treatment, with the frequency declining with continued treatment. Anti-daclizumab antibodies were transient in some patients and persistent in others.
- REMS program: [US Boxed Warning]: Due to the risks of hepatotoxicity (including autoimmune hepatitis) and other immune-mediated disorders, daclizumab is only available through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ZINBRYTA REMS Program. Prescribers and pharmacies must be certified with the program and patients must be enrolled in the program and comply with ongoing monitoring requirements. Further information is available at 1-800-456-2255.
Monitoring Parameters
ALT, AST, and total bilirubin (prior to treatment, then monthly during treatment [prior to the next dose] and for 6 months after the last dose). Screen patients for hepatitis B and C (prior to treatment initiation). Evaluate for tuberculosis (in patients at high risk) prior to treatment. Evaluate immunization status prior to treatment. Monitor for signs/symptoms of colitis, depression, dermatologic toxicity, hepatic dysfunction, hypersensitivity, immune-mediated disorders, and infection.
Pregnancy
Pregnancy Considerations
Information related to the use of daclizumab in pregnancy is limited (Gold 2016).
In general, disease-modifying therapies for multiple sclerosis are stopped prior to a planned pregnancy, and not initiated during pregnancy, except in females at high risk of multiple sclerosis activity (AAN [Rae-Grant 2018]). Consider use of agents other than daclizumab for females at high risk of disease reactivation who are planning a pregnancy. Delaying pregnancy is recommended for females with persistent high disease activity; when disease-modifying therapy is needed in these patients, other agents are preferred (ECTRIMS/EAN [Montalban 2018]).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience sore throat, stuffy nose, signs of common cold, or flu-like symptoms. Have patient report immediately to prescriber signs of liver problems (dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin), skin reaction, skin irritation, swollen glands, bloody stools, severe diarrhea, abdominal pain, signs of infection, or signs of depression (thoughts of suicide, anxiety, emotional instability, or confusion) (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.