Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet Extended Release 12 Hour, Oral:
Ampyra: 10 mg
Generic: 10 mg
Pharmacology
Mechanism of Action
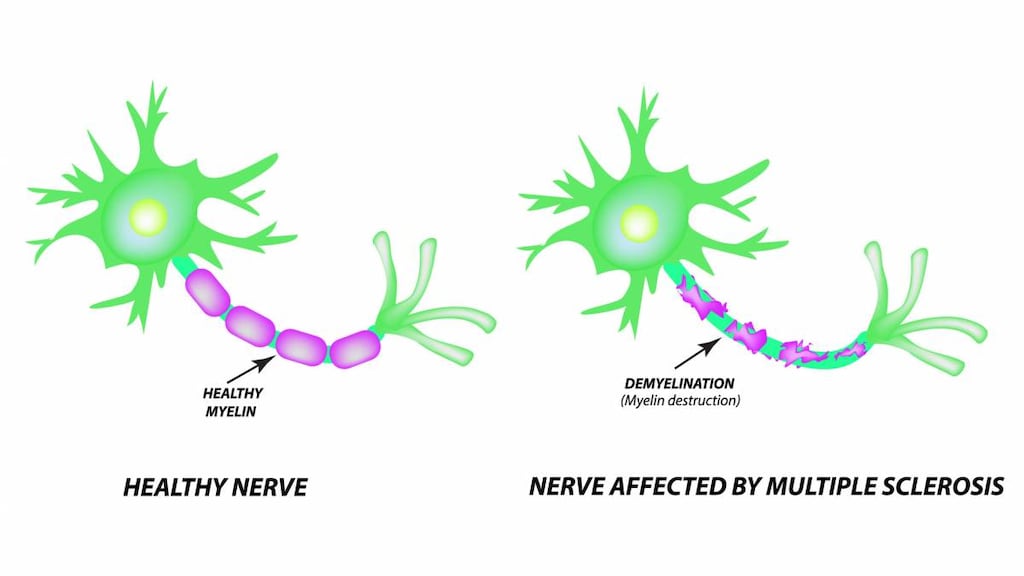
Nonspecific potassium channel blocker which improves conduction in focally demyelinated axons by delaying repolarization and prolonging the duration of action potentials. Enhanced neuronal conduction is thought to strengthen skeletal muscle fiber twitch activity, thereby, improving peripheral motor neurologic function.
Pharmacokinetics/Pharmacodynamics
Absorption
Rapid and complete
Distribution
Vd: 2.6 L/kg
Metabolism
Limited metabolism; in vitro data suggests hepatic metabolism to 3-hydroxy-4-aminopyridine occurs primarily via CYP2E1; further conjugated to 3-hydroxy-4-aminopyridine sulfate; metabolites are inactive
Excretion
Urine (96%; 90% of total dose as unchanged drug); feces (0.5%)
Time to Peak
Plasma: 3-4 hours
Half-Life Elimination
5.2-6.5 hours; prolonged in severe renal impairment (~3 times longer)
Protein Binding
Negligible; predominantly unbound to plasma proteins
Use in Specific Populations
Special Populations: Renal Function Impairment
Total body clearance of dalfampridine is reduced by ~45% in patients with mild renal impairment (CrCl 51-80 mL/minute), by ~50% in patients with moderate renal impairment (CrCl 30-50 mL/minute), and by ~75% in patients with severe renal impairment (CrCl <30 mL/minute).
Special Populations: Elderly
Clearance modestly decreased with increasing age, but not significantly enough to necessitate a dose modification.
Special Populations: Gender
A pharmacokinetic analysis suggested that women would be expected to have slightly higher Cmax than men.
Use: Labeled Indications
Treatment to improve walking in patients with multiple sclerosis (MS)
Contraindications
Hypersensitivity to dalfampridine, 4-aminopyridine, or any component of the formulation; history of seizure; moderate or severe renal impairment (CrCl ≤50 mL/minute)
Canadian labeling: Additional contraindications (not in US labeling): Mild, moderate, severe renal impairment (CrCl ≤80 mL/minute); concomitant use with compounded 4-aminopyridine or other forms of fampridine; concomitant use with drugs that inhibit organic cation transporter 2 (OCT2), such as cimetidine or quinidine
Dosage and Administration
Dosing: Adult
Multiple sclerosis: Oral: 10 mg every 12 hours (maximum daily dose: 20 mg); no additional benefit seen with doses >20 mg daily
Missed doses: Do not administer double or extra doses if a dose is missed.
Dosing: Geriatric
Refer to adult dosing.
Administration
May be administered with or without food. Do not chew, crush, dissolve, or divide tablet.
Dietary Considerations
May be taken with or without food.
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Dalfampridine Images
Drug Interactions
Cimetidine: May increase the serum concentration of Dalfampridine. Management: Recommendations differ significantly between international labelings in regards to the concomitant use of dalfampridine (referred to as fampridine in Canada) and cimetidine. Consult appropriate product labeling. Monitor therapy
MetFORMIN: May increase the serum concentration of Dalfampridine. Dalfampridine may increase the serum concentration of MetFORMIN. Monitor therapy
QuiNIDine: May increase the serum concentration of Dalfampridine. Management: Recommendations differ significantly between international labelings in regards to the concomitant use of dalfampridine (referred to as fampridine in Canada) and quinidine. Consult appropriate product labeling. Monitor therapy
Adverse Reactions
>10%: Genitourinary: Urinary tract infection (12%)
1% to 10%:
Central nervous system: Insomnia (9%), dizziness (7%), headache (7%), equilibrium disturbance (5%), paresthesia (4%)
Gastrointestinal: Nausea (7%), constipation (3%), dyspepsia (2%)
Neuromuscular & skeletal: Weakness (7%), back pain (5%), acute exacerbations of multiple sclerosis (4%)
Respiratory: Nasopharyngitis (4%), pharyngolaryngeal pain (2%)
<1%, postmarketing and/or case reports: Hypersensitivity reaction, seizure, vomiting
Warnings/Precautions
Concerns related to adverse reactions:
- Anaphylaxis: May cause anaphylaxis or severe allergic reactions; symptoms have included respiratory compromise, urticaria, and angioedema (throat and tongue). Discontinue immediately and administer appropriate medical care if a severe allergic reaction occurs.
- Seizures: Associated with a dose-dependent risk of seizure; seizures may occur within days to weeks after treatment initiation and have been reported more frequently in patients with no history of seizures. Discontinue use and do not reinitiate therapy if seizure occurs during treatment. Use is contraindicated in patients with a history of seizures. Assess risk of seizure prior to treatment initiation; use caution or avoid in patients who may have a lower seizure threshold due to predisposing factors.
- Urinary tract infection: Urinary tract infections were reported more frequently in patients receiving dalfampridine (compared to placebo),
Disease-related concerns:
- Renal impairment: Use in renal impairment is associated with an increased risk of seizure and other adverse events, primarily neurologic effects, due to increased serum concentrations; elimination is predominately via the kidneys as unchanged drug. US labeling contraindicates use in moderate-to-severe renal impairment (CrCl ≤50 mL/minute). Canadian labeling contraindicates use in mild, moderate, and severe renal impairment (CrCl ≤80 mL/minute).
Concurrent drug therapy issues:
- 4-aminopyridine formulations: Sustained release products available in the United States (dalfampridine) or in Canada (fampridine) should not be administered with other 4-aminopyridine formulations (eg, compounded immediate release fampridine).
Other warnings/precautions:
- Name confusion: The chemical entity of 4-aminopyridine is referred to with a generic name of dalfampridine in the US and with a generic name of fampridine in Canada.
Monitoring Parameters
Renal function (baseline and at least annually thereafter); EEG; walking ability
Pregnancy
Pregnancy Considerations
Information related to the use of dalfampridine in pregnancy is limited (Maillart 2016).
Patient Education
What is this drug used for?
- It is used to treat MS (multiple sclerosis).
Frequently reported side effects of this drug
- Nausea
- Headache
- Loss of strength and energy
- Trouble sleeping
- Back pain
- Stuffy nose
- Sore throat
- Constipation
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Urinary tract infection like blood in the urine, burning or painful urination, passing a lot of urine, fever, lower abdominal pain, or pelvic pain.
- Severe dizziness
- Passing out
- Severe loss of strength and energy
- Seizures
- Change in balance
- Shortness of breath
- Burning or numbness feeling
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.