Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Capsule Delayed Release, Oral:
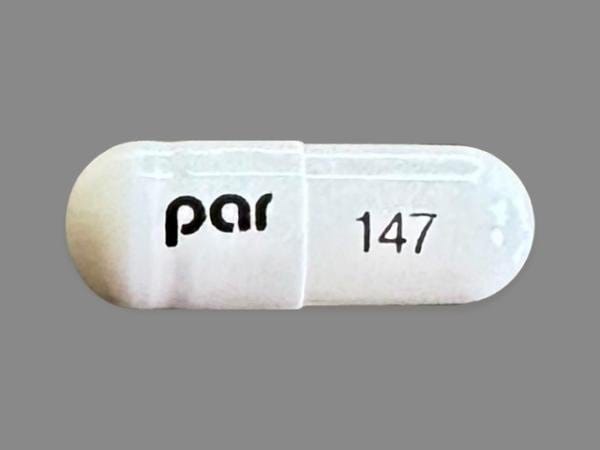
Dexilant: 30 mg, 60 mg [contains fd&c blue #2 aluminum lake]
Pharmacology
Mechanism of Action
Proton pump inhibitor; decreases acid secretion in gastric parietal cells through inhibition of (H+, K+)-ATPase enzyme system, blocking the final step in gastric acid production
Pharmacokinetics/Pharmacodynamics
Distribution
Vd: Symptomatic GERD: 40 L
Metabolism
Hepatic (extensive) via CYP2C19-mediated hydroxylation and CYP3A4-mediated oxidation; followed by reduction to sulfate, glucuronide, and glutathione conjugates (inactive)
Excretion
Urine (~51% as metabolites); feces (~48%)
Time to Peak
Serum: Two distinct peaks secondary to dual release formulation: Initial peak between 1 and 2 hours and a second higher peak between 4 and 5 hours.
Half-Life Elimination
~1 to 2 hours
Protein Binding
96% to 99%
Use in Specific Populations
Special Populations: Hepatic Function Impairment
Plasma exposure to the drug is ~2 times greater in patients with moderate hepatic impairment (Child-Pugh class B) compared with subjects with normal hepatic function.
Special Populations: Elderly
The elimination half-life is increased and AUC is higher in the elderly.
Special Populations: Gender
Systemic exposure is 43% higher in females than in males.
Use: Labeled Indications
Erosive esophagitis: Healing of all grades of erosive esophagitis in patients ≥12 years of age for up to 8 weeks; to maintain healing of erosive esophagitis and relief of heartburn for up to 6 months in adults and 16 weeks in patients 12 to 17 years of age.
Gastroesophageal reflux disease: Treatment of heartburn associated with symptomatic nonerosive gastroesophageal reflux disease (GERD) in patients ≥12 years of age for 4 weeks.
Use: Off Label
Stress ulcer prophylaxis in critically ill patientsyes
Based on the Surviving Sepsis Campaign International Guidelines for the Management of Severe Sepsis and Septic Shock, stress ulcer prophylaxis using a proton pump inhibitor or a histamine H2-receptor antagonist is recommended in sepsis or septic shock patients who have GI bleeding risk factors.
Contraindications
Known hypersensitivity (eg, anaphylaxis, acute interstitial nephritis) to dexlansoprazole or any component of the formulation; concomitant use with products that contain rilpivirine.
Dosage and Administration
Dosing: Adult
Note: Doses >30 mg do not provide additional benefit during maintenance phase.
Erosive esophagitis: Oral:
Healing: 60 mg once daily for up to 8 weeks
Maintenance: 30 mg once daily
Gastroesophageal reflux disease, symptomatic: Oral: 30 mg once daily for 4 weeks
Missed doses: If a dose is missed, administer as soon as possible; however, if the next scheduled dose is due, take the next dose on time and do not take the missed dose (do not take 2 doses at one time to make up for a missed dose).
Discontinuation of therapy: Oral: Some experts recommend a step-down approach in order to avoid worsening or rebound symptoms. One strategy is to decrease the dose by 50% over 2 to 4 weeks. If the patient is already on the lowest possible dose, alternate day therapy may be considered. If symptoms worsen during treatment or after discontinuation, patient should be re-evaluated (Kim 2018).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Note: Doses >30 mg do not provide additional benefit during maintenance phase.
Erosive esophagitis: Oral:
Healing: Children ≥12 years of age and Adolescents: Refer to adult dosing.
Maintenance: Children ≥12 years of age and Adolescents ≤17 years: 30 mg once daily for up to 16 weeks.
Gastroesophageal reflux disease, symptomatic: Children ≥12 years of age and Adolescents: Oral: Refer to adult dosing.
Missed doses: Refer to adult dosing.
Administration
Administer without regard to meals; capsules should be swallowed whole; do not chew. Alternatively, patients who are unable to swallow capsules may open the capsule, sprinkle the intact granules onto 1 tablespoon of applesauce, and swallow intact granules immediately (do not chew granules). Do not save applesauce and granule mixture for later use. Capsules may also be opened for administration via NG tube or oral syringe.
NG tube (≥16 French): Open capsules and mix granules (not crushed) with 20 mL of water. Withdraw entire mixture into catheter-tip syringe; swirl syringe gently to prevent granules from settling and administer mixture immediately through NG tube (≥16 French) into the stomach. Refill syringe with 10 mL of water, swirl gently, and flush NG tube; repeat (with a second rinse). Do not save water and granule mixture for later use.
Oral syringe: Open capsules and mix granules (not crushed) with 20 mL of water. Withdraw entire mixture into oral syringe; swirl syringe gently to prevent granules from settling and administer mixture immediately into the mouth. Refill syringe with 10 mL of water, swirl gently, and administer; repeat (with a second rinse). Do not save water and granule mixture for later use.
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted to 15°C to 30°C (59°F to 86°F).
Dexlansoprazole Images
Drug Interactions
Acalabrutinib: Proton Pump Inhibitors may decrease the serum concentration of Acalabrutinib. Avoid combination
Alcohol (Ethyl): May decrease the serum concentration of Dexlansoprazole. Avoid combination
Amphetamine: Proton Pump Inhibitors may increase the absorption of Amphetamine. Monitor therapy
Atazanavir: Proton Pump Inhibitors may decrease the serum concentration of Atazanavir. Management: See full drug interaction monograph for details. Consider therapy modification
Bisphosphonate Derivatives: Proton Pump Inhibitors may diminish the therapeutic effect of Bisphosphonate Derivatives. Monitor therapy
Bosutinib: Proton Pump Inhibitors may decrease the serum concentration of Bosutinib. Management: Consider alternatives to proton pump inhibitors, such as short-acting antacids or histamine-2 receptor antagonists. Administer alternative agents more than 2 hours before or after bosutinib. Consider therapy modification
Capecitabine: Proton Pump Inhibitors may diminish the therapeutic effect of Capecitabine. Monitor therapy
Cefditoren: Proton Pump Inhibitors may decrease the serum concentration of Cefditoren. Management: If possible, avoid use of cefditoren with proton pump inhibitors (PPIs). Consider alternative methods to minimize/control acid reflux (eg, diet modification) or alternative antimicrobial therapy if use of PPIs can not be avoided. Consider therapy modification
Cefpodoxime: Proton Pump Inhibitors may decrease the serum concentration of Cefpodoxime. Monitor therapy
Cefuroxime: Proton Pump Inhibitors may decrease the absorption of Cefuroxime. Avoid combination
Cysteamine (Systemic): Proton Pump Inhibitors may diminish the therapeutic effect of Cysteamine (Systemic). Monitor therapy
Dacomitinib: Proton Pump Inhibitors may decrease the serum concentration of Dacomitinib. Management: Avoid concurrent use of dacomitinib with proton pump inhibitors. Antacids may be used. Histamine H2-receptor antagonists (HR2A) may be used if dacomitinib is given at least 6 hours before or 10 hours after the H2RA. Avoid combination
Dasatinib: Proton Pump Inhibitors may decrease the serum concentration of Dasatinib. Management: Antacids (taken 2 hours before or after dasatinib administration) can be used in place of the proton pump inhibitor if some acid-reducing therapy is needed. Avoid combination
Delavirdine: Proton Pump Inhibitors may decrease the serum concentration of Delavirdine. Management: Chronic therapy with proton pump inhibitors (PPIs) should be avoided in patients treated with delavirdine. The clinical significance of short-term PPI therapy with delavirdine is uncertain, but such therapy should be undertaken with caution. Avoid combination
Dexmethylphenidate: Proton Pump Inhibitors may increase the absorption of Dexmethylphenidate. Specifically, proton pump inhibitors may interfere with the normal release of drug from the extended-release capsules (Focalin XR brand), which could result in both increased absorption (early) and decreased delayed absorption. Monitor therapy
Dextroamphetamine: Proton Pump Inhibitors may increase the absorption of Dextroamphetamine. Specifically, the dextroamphetamine absorption rate from mixed amphetamine salt extended release (XR) capsules may be increased in the first hours after dosing. Monitor therapy
Doxycycline: Proton Pump Inhibitors may decrease the bioavailability of Doxycycline. Monitor therapy
Erlotinib: Proton Pump Inhibitors may decrease the serum concentration of Erlotinib. Avoid combination
Fluconazole: May increase the serum concentration of Proton Pump Inhibitors. Monitor therapy
Gefitinib: Proton Pump Inhibitors may decrease the serum concentration of Gefitinib. Management: Avoid use of proton pump inhibitors (PPIs) with gefitinib when possible. If required, administer gefitinib 12 hours after administration of the PPI or 12 hours before the next dose of the PPI. Consider therapy modification
Indinavir: Proton Pump Inhibitors may decrease the serum concentration of Indinavir. Monitor therapy
Iron Preparations: Proton Pump Inhibitors may decrease the absorption of Iron Preparations. Exceptions: Ferric Carboxymaltose; Ferric Citrate; Ferric Derisomaltose; Ferric Gluconate; Ferric Hydroxide Polymaltose Complex; Ferric Pyrophosphate Citrate; Ferumoxytol; Iron Dextran Complex; Iron Sucrose. Monitor therapy
Itraconazole: Proton Pump Inhibitors may increase the serum concentration of Itraconazole. Proton Pump Inhibitors may decrease the serum concentration of Itraconazole. Management: Exposure to Tolsura brand itraconazole may be increased by PPIs; consider itraconazole dose reduction. Exposure to Sporanox brand itraconazole capsules may be decreased by PPIs. Give Sporanox brand itraconazole at least 2 hrs before or 2 hrs after PPIs Consider therapy modification
Ketoconazole (Systemic): Proton Pump Inhibitors may decrease the absorption of Ketoconazole (Systemic). Ketoconazole (Systemic) may increase the serum concentration of Proton Pump Inhibitors. Management: Administer ketoconazole with an acidic beverage, such as non-diet cola, to increase gastric acidity and improve absorption if concomitant use with proton pump inhibitors is necessary. Consider therapy modification
Ledipasvir: Proton Pump Inhibitors may decrease the serum concentration of Ledipasvir. Management: PPI doses equivalent to omeprazole 20 mg or lower may be given with ledipasvir under fasted conditions. Administration with higher doses of PPIs, 2 hours after a PPI, or in combination with food and PPIs may reduce ledipasvir bioavailability. Consider therapy modification
Lumacaftor and Ivacaftor: May decrease the serum concentration of Proton Pump Inhibitors. Monitor therapy
Mesalamine: Proton Pump Inhibitors may diminish the therapeutic effect of Mesalamine. Proton pump inhibitor-mediated increases in gastrointestinal pH may cause the premature release of mesalamine from specific sustained-release mesalamine products. Management: Consider avoiding concurrent administration of high-dose proton pump inhibitors (PPIs) with sustained-release mesalamine products. Consider therapy modification
Methotrexate: Proton Pump Inhibitors may increase the serum concentration of Methotrexate. Monitor therapy
Methylphenidate: Proton Pump Inhibitors may increase the absorption of Methylphenidate. Specifically, proton pump inhibitors may interfere with the normal release of drug from the extended-release capsules (Ritalin LA brand), which could result in both increased absorption (early) and decreased delayed absorption. Monitor therapy
Multivitamins/Minerals (with ADEK, Folate, Iron): Proton Pump Inhibitors may decrease the serum concentration of Multivitamins/Minerals (with ADEK, Folate, Iron). Specifically, the absorption of iron may be decreased. Monitor therapy
Mycophenolate: Proton Pump Inhibitors may decrease the serum concentration of Mycophenolate. Specifically, concentrations of the active mycophenolic acid may be reduced. Monitor therapy
Nelfinavir: Proton Pump Inhibitors may decrease serum concentrations of the active metabolite(s) of Nelfinavir. Proton Pump Inhibitors may decrease the serum concentration of Nelfinavir. Avoid combination
Neratinib: Proton Pump Inhibitors may decrease the serum concentration of Neratinib. Specifically, proton pump inhibitors may reduce neratinib absorption. Avoid combination
Nilotinib: Proton Pump Inhibitors may decrease the serum concentration of Nilotinib. Management: Avoid this combination when possible since separation of doses is not likely to be an adequate method of minimizing the interaction. Consider therapy modification
PAZOPanib: Proton Pump Inhibitors may decrease the serum concentration of PAZOPanib. Avoid combination
Pexidartinib: Proton Pump Inhibitors may decrease the serum concentration of Pexidartinib. Management: If acid-reduction is needed, consider administering an antacid 2 hours before or after pexidartinib, or administer pexidartinib 2 hours before or 10 hours after an H2 receptor antagonist. Avoid combination
Posaconazole: Proton Pump Inhibitors may decrease the serum concentration of Posaconazole. Consider therapy modification
Raltegravir: Proton Pump Inhibitors may increase the serum concentration of Raltegravir. Monitor therapy
Rilpivirine: Proton Pump Inhibitors may decrease the serum concentration of Rilpivirine. Avoid combination
Riociguat: Proton Pump Inhibitors may decrease the serum concentration of Riociguat. Monitor therapy
Risedronate: Proton Pump Inhibitors may diminish the therapeutic effect of Risedronate. Proton Pump Inhibitors may increase the serum concentration of Risedronate. This applies specifically to use of delayed-release risedronate. Consider therapy modification
Saquinavir: Proton Pump Inhibitors may increase the serum concentration of Saquinavir. Monitor therapy
Secretin: Proton Pump Inhibitors may diminish the diagnostic effect of Secretin. Specifically, use of PPIs may cause a hyperresponse in gastrin secretion in response to secretin stimulation testing, falsely suggesting gastrinoma. Management: Avoid concomitant use of proton pump inhibitors (PPIs) and secretin, and discontinue PPIs several weeks prior to secretin administration, with the duration of separation determined by the specific PPI. See full monograph for details. Consider therapy modification
SORAfenib: Proton Pump Inhibitors may decrease the absorption of SORAfenib. Monitor therapy
Tacrolimus (Systemic): Proton Pump Inhibitors may increase the serum concentration of Tacrolimus (Systemic). Management: Tacrolimus dose adjustment may be required. Rabeprazole, pantoprazole, or selected H2-receptor antagonists (i.e., ranitidine or famotidine) may be less likely to interact. Genetic testing may predict patients at highest risk. Consider therapy modification
Tipranavir: May decrease the serum concentration of Proton Pump Inhibitors. These data are derived from studies with Ritonavir-boosted Tipranavir. Monitor therapy
Velpatasvir: Proton Pump Inhibitors may decrease the serum concentration of Velpatasvir. Avoid combination
Voriconazole: May increase the serum concentration of Proton Pump Inhibitors. Proton Pump Inhibitors may increase the serum concentration of Voriconazole. Monitor therapy
Test Interactions
Decreases in gastric acidity may result in secondary elevation of serum chromogranin A (CgA) levels. The increased CgA level may cause false-positive results in the diagnosis of a neuroendocrine tumor. Temporarily stop dexlansoprazole at least 14 days prior to assessing CgA level; repeat level if initially elevated; use the same laboratory for all testing of CgA levels (reference ranges may vary).
Adverse Reactions
Incidence reported for adults unless otherwise specified.
1% to 10%:
Cardiovascular: Angina pectoris (<2%), bradycardia (<2%), cardiac arrhythmia (<2%), chest pain (<2%), deep vein thrombosis (<2%), edema (<2%; including oral, facial, and pharyngeal), hypertension (<2%), palpitations (<2%), tachycardia (<2%)
Central nervous system: Headache (adolescents: ≥5%; adults: <2%), abnormal dreams (<2%), anxiety (<2%), chills (<2%), depression (<2%), dizziness (<2%), falling (<2%), feeling abnormal (<2%), insomnia (<2%), memory impairment (<2%), migraine (<2%), myocardial infarction (<2%), pain (<2%), painful defecation (<2%), procedural pain (<2%), psychomotor agitation (<2%), seizure (<2%), trigeminal neuralgia (<2%), vertigo (<2%)
Dermatologic: Acne vulgaris (<2%), dermatitis (<2%), erythema (<2%), pruritus (<2%), skin lesion (<2%), skin rash (<2%), sunburn (<2%), urticaria (<2%)
Endocrine & metabolic: Change in libido (<2%), goiter (<2%), heavy menstrual bleeding (<2%), hot flash (<2%), hypercalcemia (<2%), hypokalemia (<2%), increased gastrin (<2%), increased serum glucose (<2%), increased serum potassium (<2%), increased serum total protein (<2%), menstrual disease (<2%), weight gain (<2%)
Gastrointestinal: Abdominal pain (adolescents: ≥5%), diarrhea (adolescents and adults: ≥5%), flatulence (1% to 3%), abdominal distress (<2%), abdominal tenderness (<2%), abnormal bowel sounds (<2%), abnormal stools (<2%), anorectal pain (<2%), Barrett esophagus (<2%), bezoar formation (<2%), biliary colic (<2%), change in appetite (<2%), cholelithiasis (<2%), colitis (microscopic; <2%), colonic polyps (<2%), constipation (<2%), delayed gastric emptying (<2%), duodenitis (<2%), dysgeusia (<2%), dyspepsia (<2%), dysphagia (<2%), enteritis (<2%), eructation (<2%), esophagitis (<2%), gastric polyp (<2%), gastritis (<2%), gastroenteritis (<2%), gastroesophageal reflux disease (<2%), gastrointestinal disease (<2%), gastrointestinal hypermotility (<2%), gastrointestinal perforation (<2%), gastrointestinal ulcer (<2%), halitosis (<2%), hematemesis (<2%), hematochezia (<2%), hemorrhoids (<2%), hiccups (<2%), irritable bowel syndrome (<2%), mucosal inflammation (<2%), mucus stools (<2%), oral bullae (<2%), oral herpes simplex infection (<2%), oral paresthesia (<2%), proctitis (<2%), retching (<2%), sore throat (<2%), vomiting (2%), xerostomia (<2%)
Genitourinary: Dysmenorrhea (<2%), dyspareunia (<2%), dysuria (<2%), urinary urgency (<2%), vulvovaginal infection (<2%)
Hematologic & oncologic: Anemia (<2%), decreased platelet count (<2%), lymphadenopathy (<2%), rectal hemorrhage (<2%)
Hepatic: Abnormal hepatic function tests (<2%), decreased serum bilirubin (<2%), hepatomegaly (<2%), increased serum alkaline phosphatase (<2%), increased serum alanine aminotransferase (<2%), increased serum aspartate aminotransferase (<2%), increased serum bilirubin (<2%)
Hypersensitivity: Hypersensitivity reaction (<2%)
Infection: Candidiasis (<2%), influenza (<2%), viral infection (<2%)
Neuromuscular & skeletal: Arthralgia (<2%), arthritis (<2%), asthenia (<2%), bone fracture (<2%), joint sprain (<2%), muscle cramps (<2%), musculoskeletal pain (<2%), myalgia (<2%), tremor (<2%)
Ophthalmic: Eye irritation (<2%), swelling of eye (<2%)
Otic: Otalgia (<2%), tinnitus (<2%)
Renal: Increased serum creatinine (<2%)
Respiratory: Nasopharyngitis (adolescents: ≥5%; adults: <2%), oropharyngeal pain (adolescents: ≥5%), upper respiratory tract infection (2% to 3%), asthma (<2%), bronchitis (<2%), cough (<2%), dyspnea (<2%), hyperventilation (<2%), pharyngitis (<2%), pulmonary aspiration (<2%), respiratory congestion (<2%), sinusitis (<2%)
Miscellaneous: Fever (<2%), inflammation (<2%), nodule (<2%)
<1%, postmarketing, and/or case reports: Acute renal failure, anaphylactic shock, autoimmune hemolytic anemia, blurred vision, cerebrovascular accident, chronic renal failure (Lazarus 2016), Clostridioides (formerly Clostridium) difficile-associated diarrhea, constriction of the pharynx, deafness, exfoliative dermatitis, hepatitis, hepatotoxicity (idiosyncratic) (Chalasani 2014), hypersensitivity angiitis, hypomagnesemia, hyponatremia, immune thrombocytopenia, pancreatitis, polyp (fundic gland), Stevens-Johnson syndrome, toxic epidermal necrolysis, transient ischemic attacks
Warnings/Precautions
Concerns related to adverse effects:
- Carcinoma: No occurrences of enterochromaffin-like (ECL) cell carcinoids, dysplasia, or neoplasia (such as those seen in studies of rodents exposed to lansoprazole) have been reported in humans.
- Clostridioides (formerly Clostridium) difficile-associated diarrhea (CDAD): Use of proton pump inhibitors (PPIs) may increase risk of CDAD, especially in hospitalized patients; consider CDAD diagnosis in patients with persistent diarrhea that does not improve. Use the lowest dose and shortest duration of PPI therapy appropriate for the condition being treated.
- Cutaneous and systemic lupus erythematosus: Has been reported as new onset or exacerbation of existing autoimmune disease; most cases were cutaneous lupus erythematosus (CLE), most commonly, subacute CLE (occurring within weeks to years after continuous therapy). Systemic lupus erythematosus (SLE) is less common (typically occurs within days to years after initiating treatment) and occurred primarily in young adults up to elderly patients. Discontinue therapy if signs or symptoms of CLE or SLE occur and refer to specialist for evaluation; most patients improve 4 to 12 weeks after discontinuation of dexlansoprazole.
- Fractures: Increased incidence of osteoporosis-related bone fractures of the hip, spine, or wrist may occur with PPI therapy. Patients on high-dose (multiple daily doses) or long-term therapy (≥1 year) should be monitored. Use the lowest effective dose for the shortest duration of time, use vitamin D and calcium supplementation, and follow appropriate guidelines to reduce risk of fractures in patients at risk.
- Fundic gland polyps: Use of PPIs increases risk of fundic gland polyps, especially with long term use (>1 year). May occur without symptoms but nausea, vomiting, or abdominal pain may occur; GI bleeding and/or anemia may occur with ulcerated polyps. Diagnosis of polyps may also increase risk for small intestinal blockage. Use the lowest dose and shortest duration of PPI therapy appropriate for the condition being treated.
- Hypomagnesemia: Reported rarely, usually with prolonged PPI use of ≥3 months (most cases >1 year of therapy). May be symptomatic or asymptomatic; severe cases may cause tetany, seizures, and cardiac arrhythmias. Consider obtaining serum magnesium concentrations prior to beginning long-term therapy, especially if taking concomitant digoxin, diuretics, or other drugs known to cause hypomagnesemia; and periodically thereafter. Hypomagnesemia may be corrected by magnesium supplementation, although discontinuation of dexlansoprazole may be necessary; magnesium levels typically return to normal within 1 week of stopping.
- Interstitial nephritis: Acute interstitial nephritis has been observed in patients taking PPIs; may occur at any time during therapy and is generally due to an idiopathic hypersensitivity reaction. Discontinue if acute interstitial nephritis develops.
- Vitamin B12 deficiency: Prolonged treatment (≥2 years) may lead to vitamin B12 malabsorption and subsequent vitamin B12 deficiency. The magnitude of the deficiency is dose related and the association is stronger in females and those younger in age (<30 years); prevalence is decreased after discontinuation of therapy (Lam 2013).
Disease-related concerns:
- Gastric malignancy: Relief of symptoms does not preclude the presence of a gastric malignancy.
- Gastrointestinal infection (eg, Salmonella, Campylobacter): Use of PPIs may increase risk of these infections.
- Hepatic impairment: Patients with moderate hepatic impairment (Child-Pugh class B) may require dosage reductions; use is not recommended in patients with severe hepatic impairment (Child-Pugh class C).
Concurrent drug therapy issues:
- Clopidogrel: PPIs may diminish the therapeutic effect of clopidogrel, thought to be due to reduced formation of the active metabolite of clopidogrel. The manufacturer of clopidogrel recommends either avoidance of both omeprazole (even when scheduled 12 hours apart) and esomeprazole or use of a PPI with comparatively less effect on the active metabolite of clopidogrel (eg, pantoprazole). Although lansoprazole exhibits the most potent CYP2C19 inhibition in vitro (Li 2004; Ogilvie 2011), an in vivo study of extensive CYP2C19 metabolizers showed less reduction of the active metabolite of clopidogrel when administered with lansoprazole/dexlansoprazole compared to esomeprazole/omeprazole (Frelinger 2012). In contrast to these warnings, others have recommended the continued use of PPIs, regardless of the degree of inhibition, in patients with a history of GI bleeding or multiple risk factors for GI bleeding who are also receiving clopidogrel since no evidence has established clinically meaningful differences in outcome; however, a clinically significant interaction cannot be excluded in those who are poor metabolizers of clopidogrel (Abraham 2010; Levine 2011). The manufacturer of dexlansoprazole states that no dosage adjustment is necessary for clopidogrel when used concurrently with approved doses.
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Other warnings/precautions:
- Laboratory test interference: Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acid; may cause false-positive results in diagnostic investigations for neuroendocrine tumors. Temporarily stop dexlansoprazole treatment at least 14 days before CgA test; if CgA level is high, repeat test to confirm. Use same commercial laboratory for testing to prevent variable results.
Monitoring Parameters
Magnesium levels (prior to initiation of therapy and periodically thereafter) in patients on long-term treatment or those taking digoxin, diuretics, or other drugs that cause hypomagnesemia.
Pregnancy
Pregnancy Considerations
Adverse events have not been observed in animal reproduction studies.
Recommendations for the treatment of GERD in pregnancy are available. As in nonpregnant patients, lifestyle modifications followed by other medications are the initial treatments (Body 2016; Huerta-Iga 2016; Katz 2013; van der Woude 2014). Based on available data, PPIs may be used when clinically indicated (use of agents with more available data may be preferred) (Body 2016; Matok 2012; Pasternak 2010; van der Woude 2014).
Patient Education
What is this drug used for?
- It is used to treat gastroesophageal reflux disease (GERD; acid reflux).
- It is used to treat heartburn.
- It is used to treat or prevent ulcers of the swallowing tube (esophagus).
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Abdominal pain
- Nausea
- Vomiting
- Passing gas
- Headache
- Diarrhea
- Stuffy nose
- Sore throat
- Common cold
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Low magnesium like mood changes; muscle pain or weakness; muscle cramps or spasms; seizures; tremors; lack of appetite; severe nausea or vomiting; or an abnormal heartbeat.
- Kidney problems like unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain.
- Lupus like rash on the cheeks or other body parts, sunburn easy, muscle or joint pain, chest pain or shortness of breath, or swelling in the arms or legs.
- Dizziness
- Passing out
- Fast heartbeat
- Bone pain
- Clostridioides (formerly Clostridium) difficile-associated diarrhea like abdominal pain or cramps, severe diarrhea or watery stools, or bloody stools.
- Stevens-Johnson syndrome/toxic epidermal necrolysis like red, swollen, blistered, or peeling skin (with or without fever); red or irritated eyes; or sores in mouth, throat, nose, or eyes.
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.