Boxed Warning
Fetal toxicity:
When pregnancy is detected, discontinue eprosartan as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Generic: 600 mg
Pharmacology
Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Eprosartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis. Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because eprosartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Eprosartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Pharmacokinetics/Pharmacodynamics
Metabolism
Minimally hepatic
Excretion
Feces (90%); urine (7% primarily as unchanged drug)
Time to Peak
Serum: Fasting: 1 to 2 hours
Half-Life Elimination
Terminal: 5 to 9 hours (Bottorff, 1999)
Protein Binding
98%
Use in Specific Populations
Special Populations: Renal Function Impairment
AUC increased 70% to 90% and Cmax increased 30% to 50% in patients with moderate or severe renal impairment, respectively.
Special Populations: Hepatic Function Impairment
AUC increased approximately 40% in men with decreased hepatic function.
Special Populations: Elderly
AUC, Cmax, and Tmax increased approximately 2-fold.
Use: Labeled Indications
Hypertension: Management of hypertension
Use: Off Label
Acute coronary syndrome (secondary prevention of cardiovascular events)yes
Based on the 2014 American Heart Association/American College of Cardiology (AHA/ACC) guidelines for the management of patients with non-ST-elevation acute coronary syndromes (NSTE-ACS) and the 2013 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines for the management of patients with ST-elevation acute coronary syndromes (STE-ACS), an ARB is recommended and effective in patients with NSTE-ACS or STE-ACS who have indications for but are intolerant of ACE inhibitors; this includes patients with heart failure, MI, or anterior MI who have a left ventricular ejection fraction (LVEF) ≤40%. In post-STE-ACS patients, initiate within the first 24 hours.
Stable coronary artery diseaseyes
Based on the 2012 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline for the diagnosis and management of patients with stable ischemic heart disease, an ACE inhibitor or ARB should be prescribed in all patients with stable ischemic heart disease who also have hypertension, diabetes mellitus, LVEF <40%, or CKD unless contraindicated.
Contraindications
Hypersensitivity to eprosartan or any component of the formulation; coadministration with aliskiren in patients with diabetes
Documentation of allergenic cross-reactivity for angiotensin II receptor blockers is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Canadian labeling: Additional contraindications (not in US labeling): Hemodynamically significant bilateral renovascular disease or severe stenosis of a solitary functioning kidney; hereditary problems of galactose intolerance, the Lapp lactase deficiency, or glucose-galactose malabsorption; concomitant use with aliskiren in patients with moderate to severe renal impairment (GFR <60 mL/minute/1.73 m2); concomitant use with angiotensin-converting enzyme (ACE) inhibitors in patients with diabetic nephropathy; pregnancy; breastfeeding
Dosage and Administration
Dosing: Adult
Hypertension: Note: For initial treatment in patients with blood pressure ≥20/10 mm Hg above goal, may be used in combination with another appropriate agent (eg, a long-acting dihydropyridine calcium channel blocker or thiazide diuretic). For patients <20/10 mm Hg above goal, some experts recommend an initial trial of monotherapy; however, over time, many patients will require combination therapy (ACC/AHA [Whelton 2017]; Mann 2019).
Oral: Initial: 600 mg once daily; evaluate response every 4 to 6 weeks and titrate as needed based on patient response up to 800 mg/day in 1 to 2 divided doses (ACC/AHA [Whelton 2017]; Mann 2019)
Dosing: Geriatric
Refer to adult dosing.
Administration
Oral: May be administered with or without food.
Storage
Store at 20°C to 25°C (68°F to 77°F).
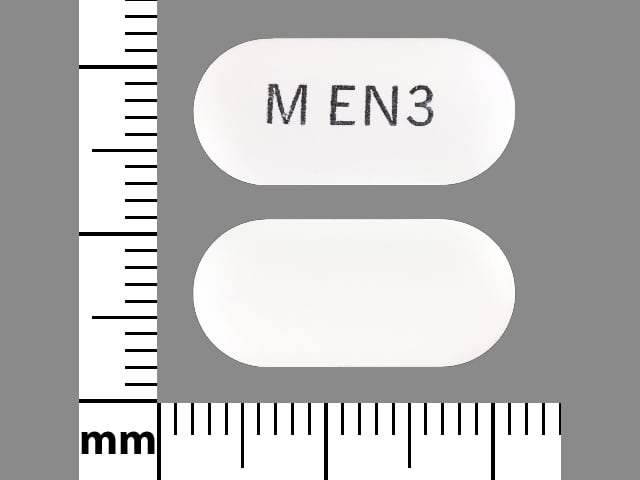
Eprosartan Images
Drug Interactions
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Aliskiren: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Aliskiren may enhance the hypotensive effect of Angiotensin II Receptor Blockers. Aliskiren may enhance the nephrotoxic effect of Angiotensin II Receptor Blockers. Management: Aliskiren use with ACEIs or ARBs in patients with diabetes is contraindicated. Combined use in other patients should be avoided, particularly when CrCl is less than 60 mL/min. If combined, monitor potassium, creatinine, and blood pressure closely. Consider therapy modification
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Angiotensin II: Receptor Blockers may diminish the therapeutic effect of Angiotensin II. Monitor therapy
Angiotensin-Converting Enzyme Inhibitors: Angiotensin II Receptor Blockers may enhance the adverse/toxic effect of Angiotensin-Converting Enzyme Inhibitors. Angiotensin II Receptor Blockers may increase the serum concentration of Angiotensin-Converting Enzyme Inhibitors. Management: In US labeling, use of telmisartan and ramipril is not recommended. It is not clear if any other combination of an ACE inhibitor and an ARB would be any safer. Consider alternatives to the combination when possible. Consider therapy modification
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
CycloSPORINE (Systemic): Angiotensin II Receptor Blockers may enhance the hyperkalemic effect of CycloSPORINE (Systemic). Monitor therapy
Dapoxetine: May enhance the orthostatic hypotensive effect of Angiotensin II Receptor Blockers. Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Drospirenone: Angiotensin II Receptor Blockers may enhance the hyperkalemic effect of Drospirenone. Monitor therapy
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
Eplerenone: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Heparin: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Heparins (Low Molecular Weight): May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Lithium: Angiotensin II Receptor Blockers may increase the serum concentration of Lithium. Management: Lithium dosage reductions will likely be needed following the addition of an angiotensin II receptor antagonist. Consider therapy modification
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: Angiotensin II Receptor Blockers may enhance the adverse/toxic effect of Nonsteroidal Anti-Inflammatory Agents. Specifically, the combination may result in a significant decrease in renal function. Nonsteroidal Anti-Inflammatory Agents may diminish the therapeutic effect of Angiotensin II Receptor Blockers. The combination of these two agents may also significantly decrease glomerular filtration and renal function. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Potassium Salts: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Potassium-Sparing Diuretics: Angiotensin II Receptor Blockers may enhance the hyperkalemic effect of Potassium-Sparing Diuretics. Monitor therapy
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Ranolazine: May enhance the adverse/toxic effect of Angiotensin II Receptor Blockers. Monitor therapy
Sodium Phosphates: Angiotensin II Receptor Blockers may enhance the nephrotoxic effect of Sodium Phosphates. Specifically, the risk of acute phosphate nephropathy may be enhanced. Management: Consider avoiding this combination by temporarily suspending treatment with ARBs, or seeking alternatives to oral sodium phosphate bowel preparation. If the combination cannot be avoided, maintain adequate hydration and monitor renal function closely. Consider therapy modification
Tacrolimus (Systemic): Angiotensin II Receptor Blockers may enhance the hyperkalemic effect of Tacrolimus (Systemic). Monitor therapy
Tolvaptan: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Trimethoprim: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
May lead to false-negative aldosterone/renin ratio (ARR) (Funder 2016).
Adverse Reactions
1% to 10%:
Cardiovascular: Chest pain (≥1%)
Central nervous system: Fatigue (2%), dizziness (≥1%), headache (≥1%), depression (1%)
Endocrine & metabolic: Dependent edema (≥1%), hypertriglyceridemia (1%)
Gastrointestinal: Abdominal pain (2%), diarrhea (≥1%), dyspepsia (≥1%)
Genitourinary: Urinary tract infection (1%)
Infection: Viral infection (2%)
Neuromuscular & skeletal: Arthralgia (2%), myalgia (≥1%)
Renal: Increased blood urea nitrogen (1%)
Respiratory: Upper respiratory tract infection (8%), pharyngitis (4%), rhinitis (4%), cough (ARBs: 3%; Matchar 2008), bronchitis (≥1%), sinusitis (≥1%)
Miscellaneous: Accidental injury (2%)
<1%, postmarketing, and/or case reports: Albuminuria, alcohol intolerance, anemia, angina pectoris, anorexia, anxiety, arthritis, asthenia, asthma, ataxia, atrial fibrillation, back pain, bradycardia, conjunctivitis, constipation, cystitis, decreased hemoglobin (>20% decrease), diabetes mellitus, diaphoresis, drowsiness, ECG abnormality, eczema, epistaxis, esophagitis, exacerbation of arthritis, extrasystoles, facial edema, fever, flatulence, flu-like symptoms, flushing sensation, furunculosis, gastritis, gastroenteritis, gingivitis, glycosuria, gout, hematuria, herpes simplex infection, hypercholesterolemia, hyperglycemia, hyperkalemia, hypokalemia, hyponatremia, hypotension, increased creatine phosphokinase, increased serum alanine aminotransferase, increased serum aspartate aminotransferase, insomnia, lower limb cramp, maculopapular rash, malaise, migraine, nausea, nephrolithiasis, nervousness, neuritis, nonthrombocytopenic purpura, orthostatic hypotension, osteoarthritis, otitis externa, otitis media, pain, palpitations, paresthesia, periodontitis, peripheral edema, peripheral ischemia, polyuria, pruritus, rigors, skeletal pain, skin rash, substernal pain, tachycardia, tendonitis, thrombocytopenia, tinnitus, toothache, tremor, urinary frequency, urinary incontinence, vertigo, visual disturbance, vomiting, xerophthalmia, xerostomia
Warnings/Precautions
Concerns related to adverse effects:
- Angioedema: Angioedema has been reported rarely with some angiotensin II receptor antagonists (ARBs) and may occur at any time during treatment (especially following first dose). It may involve the head and neck (potentially compromising airway) or the intestine (presenting with abdominal pain). Patients with idiopathic or hereditary angioedema or previous angioedema associated with ACE-inhibitor therapy may be at an increased risk. Prolonged frequent monitoring may be required, especially if tongue, glottis, or larynx are involved, as they are associated with airway obstruction. Patients with a history of airway surgery may have a higher risk of airway obstruction. Discontinue therapy immediately if angioedema occurs. Aggressive early management is critical. Intramuscular (IM) administration of epinephrine may be necessary. Do not readminister to patients who have had angioedema with ARBs.
- Hyperkalemia: May occur; risk factors include renal dysfunction, diabetes mellitus, concomitant use of ACE inhibitors, aliskiren, potassium-sparing diuretics, potassium supplements and/or potassium containing salts. Use cautiously, if at all, with these agents and monitor potassium closely.
- Hypotension: Symptomatic hypotension may occur upon initiation in patients who are salt- or volume-depleted (eg, those treated with high-dose diuretics); correct volume depletion prior to administration. This transient hypotensive response is not a contraindication to further treatment with eprosartan.
- Renal function deterioration: May be associated with deterioration of renal function and/or increases in serum creatinine, particularly in patients with low renal blood flow (eg, renal artery stenosis, heart failure) whose glomerular filtration rate (GFR) is dependent on efferent arteriolar vasoconstriction by angiotensin II; deterioration may result in oliguria, acute renal failure, and progressive azotemia. Small increases in serum creatinine may occur following initiation; consider discontinuation only in patients with progressive and/or significant deterioration in renal function.
Disease-related concerns:
- Aortic/mitral stenosis: Use with caution in patients with significant aortic/mitral stenosis.
- Ascites: Avoid use in patients with ascites due to cirrhosis or refractory ascites; if use cannot be avoided in patients with ascites due to cirrhosis, monitor blood pressure and renal function carefully to avoid rapid development of renal failure (AASLD [Runyon 2012]).
- Renal artery stenosis: Use eprosartan with caution in patients with unstented unilateral/bilateral renal artery stenosis. When unstented bilateral renal artery stenosis is present, use is generally avoided due to the elevated risk of deterioration in renal function unless possible benefits outweigh risks.
- Renal impairment: Use with caution in preexisting renal insufficiency and severe renal impairment.
Concurrent drug therapy issues:
- Angiotensin-converting enzyme (ACE) inhibitors and renin inhibitors: Concomitant use of an ACE-inhibitor or renin inhibitor (eg, aliskiren) is associated with an increased risk of hypotension, hyperkalemia, and renal dysfunction. Concomitant use with aliskiren should be avoided in patients with GFR <60 mL/minute and is contraindicated in patients with diabetes mellitus (regardless of GFR).
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pregnancy: [US Boxed Warning]: Drugs that act on the renin-angiotensin system can cause injury and death to the developing fetus. Discontinue as soon as possible once pregnancy is detected.
- Surgical patients: In patients on chronic angiotensin receptor blocker (ARB) therapy, intraoperative hypotension may occur with induction and maintenance of general anesthesia; however, discontinuation of therapy prior to surgery is controversial. If continued preoperatively, avoidance of hypotensive agents during surgery is prudent (Hillis 2011). Based on current research and clinical guidelines in patients undergoing non-cardiac surgery, continuing angiotensin-receptor blockers (ARB) is reasonable in the perioperative period. If ARBs are held before surgery, it is reasonable to restart postoperatively as soon as clinically feasible (ACC/AHA [Fleisher 2014]).
Monitoring Parameters
Blood pressure; serum potassium, serum creatinine, BUN, and eGFR (before and after dose changes and at least annually thereafter)
Hypertension: The 2017 guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults (ACC/AHA [Whelton 2017]):
Confirmed hypertension and known CVD or 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥10%: Target blood pressure <130/80 mm Hg is recommended
Confirmed hypertension without markers of increased ASCVD risk: Target blood pressure <130/80 mm Hg may be reasonable
Diabetes and hypertension: The American Diabetes Association (ADA) guidelines (ADA 2019):
Patients 18 to 65 years of age, without ASCVD, and 10-year ASCVD risk <15%: Target blood pressure <140/90 mm Hg is recommended.
Patients 18 to 65 years of age and known ASCVD or 10-year ASCVD risk >15%: Target blood pressure <130/80 mm Hg may be appropriate if it can be safely attained.
Patients >65 years of age (healthy or complex/intermediate health): Target blood pressure <140/90 mm Hg is recommended.
Patients >65 years of age (very complex/poor health): Target blood pressure <150/90 mm Hg is recommended.
Pregnancy
Pregnancy Risk Factor
D
Pregnancy Considerations
[US Boxed Warning]: Drugs that act on the renin-angiotensin system can cause injury and death to the developing fetus. When pregnancy is detected, discontinue as soon as possible. The use of drugs which act on the renin-angiotensin system are associated with oligohydramnios. Oligohydramnios, due to decreased fetal renal function, may lead to fetal lung hypoplasia and skeletal malformations. Oligohydramnios may not appear until after irreversible fetal injury has occurred. Use in pregnancy is also associated with anuria, hypotension, renal failure, skull hypoplasia, and death in the fetus/neonate. The exposed fetus should be monitored for fetal growth, amniotic fluid volume, and organ formation. Infants exposed in utero should be monitored for hyperkalemia, hypotension, and oliguria (exchange transfusions or dialysis may be needed). These adverse events are generally associated with maternal use in the second and third trimesters.
Chronic maternal hypertension itself is also associated with adverse events in the fetus/infant. The risk of birth defects, low birth weight, premature delivery, stillbirth, and neonatal death may be increased with chronic hypertension in pregnancy. Actual risks may be related to duration and severity of maternal hypertension (ACOG 203 2019).
The use of angiotensin II receptor blockers is generally not recommended to treat chronic hypertension in pregnant women and should generally be avoided in women planning a pregnancy (ACOG 203 2019).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience common cold symptoms. Have patient report immediately to prescriber signs of kidney problems (unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain), signs of high potassium (abnormal heartbeat, confusion, dizziness, passing out, weakness, shortness of breath, or numbness or tingling feeling), severe dizziness, passing out, muscle weakness, or muscle pain (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.