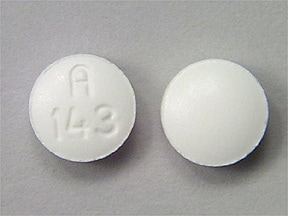
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Elixir, Oral, as sulfate:
Hyosyne: 0.125 mg/5 mL (473 mL) [contains alcohol, usp; lemon flavor]
Generic: 0.125 mg/5 mL (473 mL)
Solution, Injection, as sulfate:
Levsin: 0.5 mg/mL (1 mL [DSC]) [contains benzyl alcohol]
Solution, Injection, as sulfate [preservative free]:
Levsin: 0.5 mg/mL (1 mL)
Generic: 0.5 mg/mL (1 mL)
Solution, Oral, as sulfate:
Hyosyne: 0.125 mg/mL (15 mL) [lemon flavor]
Generic: 0.125 mg/mL (15 mL)
Tablet, Oral, as sulfate:
Levsin: 0.125 mg
Oscimin: 0.125 mg [peppermint flavor]
Generic: 0.125 mg
Tablet Disintegrating, Oral, as sulfate:
Anaspaz: 0.125 mg [scored; contains corn starch, fd&c yellow #10 (quinoline yellow), fd&c yellow #6 (sunset yellow)]
Ed-Spaz: 0.125 mg [scored]
NuLev: 0.125 mg [peppermint flavor]
Oscimin: 0.125 mg [DSC] [peppermint flavor]
Generic: 0.125 mg
Tablet Extended Release, Oral, as sulfate:
Symax Duotab: 0.375 mg [contains brilliant blue fcf (fd&c blue #1)]
Tablet Extended Release 12 Hour, Oral, as sulfate:
Levbid: 0.375 mg
Oscimin SR: 0.375 mg
Symax-SR: 0.375 mg [scored]
Generic: 0.375 mg
Tablet Sublingual, Sublingual, as sulfate:
Levsin/SL: 0.125 mg
Oscimin: 0.125 mg [peppermint flavor]
Symax-SL: 0.125 mg [mint flavor]
Generic: 0.125 mg
Pharmacology
Mechanism of Action
Blocks the action of acetylcholine at parasympathetic sites in smooth muscle, secretory glands, and the CNS; increases cardiac output, dries secretions, antagonizes histamine and serotonin
Pharmacokinetics/Pharmacodynamics
Absorption
Well absorbed
Metabolism
Hepatic
Excretion
Urine
Onset of Action
2 to 3 minutes
Duration of Action
Regular release: 4 to 6 hours; Extended release (Levbid, Symax Duotab): 8 to 12 hours
Half-Life Elimination
Regular release: 2 to 3.5 hours; Extended release (Levbid): ~7 hours
Use: Labeled Indications
Anesthesia:
Preoperative antimuscarinic: Preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions; to reduce volume and acidity of gastric secretions; to block cardiac vagal inhibitory reflexes during induction of anesthesia and intubation
Reversal of neuromuscular blockade and associated muscarinic effects: Protects against peripheral muscarinic effects (such as bradycardia and excessive secretions produced by halogenated hydrocarbons and cholinergic agents [such as physostigmine, neostigmine, and pyridostigmine]) given to reverse actions of curariform agents
Antidote for anticholinesterase agent poisoning: Antidote for poisoning by anticholinesterase agents
Biliary and renal colic: Adjunctive therapy with morphine or other opioids for the symptomatic relief of biliary and renal colic
Diagnostic procedures: Reduces GI motility to facilitate diagnostic procedures such as endoscopy or hypotonic duodenography; may also improve radiologic visibility of the kidneys
GI disorders:
Aid in the control of acute episodes of gastric secretion, visceral spasm, hypermotility in spastic colitis, pylorospasm, and associated abdominal cramps; relieve symptoms in functional intestinal disorders (eg, mild dysenteries, diverticulitis) and infant colic (elixir and oral solution)
Adjunctive therapy for treatment in peptic ulcer; irritable bowel syndrome (irritable colon, spastic colon, acute enterocolitis, mucous colitis) and other functional GI disorders; neurogenic bowel disturbances (including splenic flexure syndrome and neurogenic colon)
Pancreatitis: Reduce pain and hypersecretion in pancreatitis
Parkinsonism: In parkinsonism, to reduce rigidity and tremors and to control associated sialorrhea and hyperhidrosis
Partial heart block: For use in certain cases of partial heart block associated with vagal activity
Rhinitis: “Drying agent” in the relief of symptoms of acute rhinitis
Urinary system disorder: To control hypermotility in spastic bladder and cystitis; adjunctive therapy in the treatment of neurogenic bladder
Contraindications
Hypersensitivity to belladonna alkaloids or any component of the formulation; glaucoma; obstructive uropathy (eg, bladder neck obstruction due to prostatic hypertrophy); myasthenia gravis; obstructive GI tract disease (eg, achalasia, pyloroduodenal stenosis), paralytic ileus, intestinal atony of elderly or debilitated patients, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis; unstable cardiovascular status; myocardial ischemia. Note: Some extended release products are not recommended in children <12 years of age; refer to manufacturer's labeling.
Dosage and Administration
Dosing: Adult
Gastrointestinal disorders:
Oral:
Tablet, dispersible:
Anaspaz, ED-SPAZ, NuLev: 0.125 to 0.25 mg every 4 hours or as needed; maximum: 1.5 mg/day
Oscimin: 0.125 to 0.25 mg 3 to 4 times daily; may increase to every 4 hours as needed; maximum: 1.5 mg/day
Tablet, extended release:
Levbid: 0.375 to 0.75 mg every 12 hours; maximum: 1.5 mg/day
Oscimin SR, Symax Duotab, Symax SR: 0.375 to 0.75 mg every 12 hours or 0.375 mg every 8 hours; maximum: 1.5 mg/day
Tablet, regular release:
Levsin: 0.125 to 0.25 mg every 4 hours or as needed; maximum: 1.5 mg/day
Oscimin: 0.125 to 0.25 mg 3 to 4 times daily; may increase to every 4 hours as needed; maximum: 1.5 mg/day
Tablet, sublingual (Levsin/SL, Oscimin, Symax SL): 0.125 to 0.25 mg 3 to 4 times daily; may increase to every 4 hours as needed; maximum: 1.5 mg/day
Drops (Hyosyne [0.125 mg/mL]): 0.125 mg (1 mL) to 0.25 mg (2 mL) every 4 hours or as needed; maximum: 1.5 mg (12 mL) per day
Elixir (Hyosyne [0.125 mg/5 mL]): 0.125 mg (5 mL) to 0.25 mg (10 mL) every 4 hours or as needed; maximum: 1.5 mg (60 mL) per day
IM, IV, SubQ: 0.25 to 0.5 mg; may repeat as needed up to 4 times daily, at 4-hour intervals
Diagnostic procedures: IV: 0.25 to 0.5 mg given 5 to 10 minutes prior to procedure
Preanesthesia: IM, IV, SubQ: 5 mcg/kg given 30 to 60 minutes prior to induction of anesthesia or at the time preoperative opioids or sedatives are administered
To reduce drug-induced bradycardia during surgery: IV: 0.125 mg; repeat as needed
Reverse neuromuscular blockade: IM, IV, SubQ: 0.2 mg for every 1 mg neostigmine (or the physostigmine/pyridostigmine equivalent)
Dosing: Geriatric
Avoid use (Beers Criteria [AGS 2019]).
Dosing: Pediatric
Note: Oral dosage forms (drops and elixirs) are different (ie, 0.125 mg/mL [drops] and 0.125 mg/5 mL [solution]); precaution should be taken to verify and avoid confusion between the different concentrations; dose should be clearly presented with corresponding product formulation.
Gastrointestinal and urinary systemic spasms (including infant colic, biliary and renal colic):
Infants and Children <2 years: Oral: Drops (eg, Hyosyne [0.125 mg/mL]): Do not exceed 6 doses in 24 hours
3.4 to <5 kg: 0.01375 mg (0.11 mL or 4 drops) every 4 hours or as needed; maximum daily dose: 0.0825 mg (0.66 mL or 24 drops)/day
5 to <7 kg: 0.0175 mg (0.14 mL or 5 drops) every 4 hours or as needed; maximum daily dose: 0.105 mg (0.84 mL or 30 drops)/day
7 to <10 kg: 0.02 mg (0.16 mL or 6 drops) every 4 hours or as needed; maximum daily dose: 0.12 mg (0.96 mL or 36 drops)/day
≥10 kg: 0.0275 mg (0.22 mL or 8 drops) every 4 hours or as needed; maximum daily dose: 0.165 mg (1.32 mL or 48 drops)/day
Children 2 to <12 years: Oral:
Drops (eg, Hyosyne [0.125 mg/mL]): 0.03125 mg (0.25 mL) to 0.125 mg (1 mL) every 4 hours or as needed; maximum daily dose: 0.75 mg (6 mL)/day; do not exceed 6 doses in 24 hours
Elixir (eg, Hyosyne [0.125 mg/5 mL]): Do not exceed 6 doses in 24 hours:
10 to <20 kg: 0.03125 mg (1.25 mL) every 4 hours or as needed; maximum daily dose: 0.1875 mg (7.5 mL)/day
20 to <40 kg: 0.0625 mg (2.5 mL) every 4 hours or as needed; maximum daily dose: 0.375 mg (15 mL)/day
40 to <50 kg: 0.09375 mg (3.75 mL) every 4 hours or as needed; maximum daily dose: 0.5625 mg (22.5 mL)/day
≥50 kg: 0.125 mg (5 mL) every 4 hours or as needed; maximum daily dose: 0.75 mg (30 mL)/day
Tablets, immediate release: regular tablet [Levsin], sublingual [Levsin/SL], dispersible [Anaspaz, ED-SPAZ, NuLev]): 0.0625 to 0.125 mg every 4 hours or as needed; maximum daily dose: 0.75 mg/day
Children ≥12 years and Adolescents: Oral:
Immediate release:
Drops (Hyosyne [0.125 mg/mL]): 0.125 mg (1 mL) to 0.25 mg (2 mL) every 4 hours or as needed; maximum daily dose: 1.5 mg (12 mL)/day
Elixir (Hyosyne [0.125 mg/5 mL]): 0.125 mg (5 mL) to 0.25 mg (10 mL) every 4 hours or as needed; maximum daily dose: 1.5 mg (60 mL)/day
Tablet, dispersible:
Anaspaz, ED-SPAZ, NuLev: 0.125 to 0.25 mg every 4 hours or as needed; maximum daily dose: 1.5 mg/day
Oscimin: 0.125 to 0.25 mg 3 to 4 times daily; may increase to every 4 hours as needed; maximum daily dose: 1.5 mg/day
Tablet:
Levsin: 0.125 to 0.25 mg every 4 hours or as needed; maximum daily dose: 1.5 mg/day
Oscimin: 0.125 to 0.25 mg 3 to 4 times daily; may increase to every 4 hours as needed; maximum daily dose: 1.5 mg/day
Tablet, sublingual
Levsin/SL: 0.125 to 0.25 mg every 4 hours or as needed; maximum daily dose: 1.5 mg/day
Oscimin, Symax SL: 0.125 to 0.25 mg 3 to 4 times daily; may increase to every 4 hours as needed; maximum daily dose: 1.5 mg/day
Extended release: Tablet:
Levbid: 0.375 to 0.75 mg every 12 hours; maximum daily dose: 1.5 mg/day
Oscimin SR, Symax Duotab, Symax SR: 0.375 to 0.75 mg every 12 hours or 0.375 mg every 8 hours; maximum daily dose: 1.5 mg/day
Preanesthesia: Children >2 years and Adolescents: IM, IV, SubQ: 5 mcg/kg administered 30 to 60 minutes prior to induction of anesthesia or at the time preoperative opioids or sedatives are administered
Reduce drug-induced bradycardia during surgery: Children >2 years and Adolescents: IV: 0.125 mg; repeat as needed
Reversal of neuromuscular blockage: Children >2 years and Adolescents: IV, IM, SubQ: 0.2 mg for every 1 mg neostigmine (or physostigmine/pyridostigmine equivalent)
Administration
Oral:
Elixir (Hyosyne): Administration prior to meals (~30 to 60 minutes) is recommended (but not required) when used to treat gastrointestinal disorders.
Drops (Hyosyne): Administration prior to meals (~30 to 60 minutes) is recommended (but not required) when used to treat gastrointestinal disorders. Use provided dropper to accurately measure dose.
Tablet, regular release (Levsin, Oscimin): Administration prior to meals (~30 to 60 minutes) is recommended (but not required) when used to treat gastrointestinal disorders.
Tablet, dispersible: Administration prior to meals (~30 to 60 minutes) is recommended (but not required) when used to treat gastrointestinal disorders.
Anaspaz: Tablets may be used sublingually, chewed, or swallowed whole.
Ed-Spaz: Place on top of tongue and allow to dissolve; swallow with saliva.
NuLev, Oscimin: Tablets may be chewed or placed on tongue and allowed to disintegrate.
Tablet, extended release: Administration prior to meals (~30 to 60 minutes) is recommended (but not required) when used to treat gastrointestinal disorders.
Levbid: Do not crush or chew.
Oscimin SR, Symax Duotab, Symax SR: Swallow whole.
Tablet, sublingual: Administration prior to meals (~30 to 60 minutes) is recommended (but not required) when used to treat gastrointestinal disorders.
Levsin/SL: Tablets may be used sublingually, chewed, or swallowed whole.
Symax SL: Tablets may be used sublingually or swallowed whole.
Oscimin: Administer sublingually.
IM: May be administered without dilution.
IV: Inject over at least 1 minute. May be administered without dilution.
SubQ: May be administered without dilution.
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C and 30°C (59°F and 86°F).
Hyoscyamine Images
Drug Interactions
Acetylcholinesterase Inhibitors: May diminish the therapeutic effect of Anticholinergic Agents. Anticholinergic Agents may diminish the therapeutic effect of Acetylcholinesterase Inhibitors. Monitor therapy
Aclidinium: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Amantadine: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Antacids: May decrease the serum concentration of Hyoscyamine. Management: Administer immediate release hyoscyamine before meals and antacids after meals when these agents are given in combination. Consider therapy modification
Anticholinergic Agents: May enhance the adverse/toxic effect of other Anticholinergic Agents. Monitor therapy
Botulinum Toxin-Containing Products: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Cannabinoid-Containing Products: Anticholinergic Agents may enhance the tachycardic effect of Cannabinoid-Containing Products. Exceptions: Cannabidiol. Monitor therapy
Chloral Betaine: May enhance the adverse/toxic effect of Anticholinergic Agents. Monitor therapy
Cimetropium: Anticholinergic Agents may enhance the anticholinergic effect of Cimetropium. Avoid combination
Eluxadoline: Anticholinergic Agents may enhance the constipating effect of Eluxadoline. Avoid combination
Gastrointestinal Agents (Prokinetic): Anticholinergic Agents may diminish the therapeutic effect of Gastrointestinal Agents (Prokinetic). Monitor therapy
Glucagon: Anticholinergic Agents may enhance the adverse/toxic effect of Glucagon. Specifically, the risk of gastrointestinal adverse effects may be increased. Monitor therapy
Glycopyrrolate (Oral Inhalation): Anticholinergic Agents may enhance the anticholinergic effect of Glycopyrrolate (Oral Inhalation). Avoid combination
Glycopyrronium (Topical): May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Ipratropium (Oral Inhalation): May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Itopride: Anticholinergic Agents may diminish the therapeutic effect of Itopride. Monitor therapy
Ketoconazole (Systemic): Hyoscyamine may decrease the serum concentration of Ketoconazole (Systemic). Management: Take hyoscyamine at least 2 hours after ingestion of ketoconazole. Monitor for decreased therapeutic effects of ketoconazole if used together with hyoscyamine. Consider therapy modification
Levosulpiride: Anticholinergic Agents may diminish the therapeutic effect of Levosulpiride. Avoid combination
Mianserin: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Mirabegron: Anticholinergic Agents may enhance the adverse/toxic effect of Mirabegron. Monitor therapy
Nitroglycerin: Anticholinergic Agents may decrease the absorption of Nitroglycerin. Specifically, anticholinergic agents may decrease the dissolution of sublingual nitroglycerin tablets, possibly impairing or slowing nitroglycerin absorption. Monitor therapy
Opioid Agonists: Anticholinergic Agents may enhance the adverse/toxic effect of Opioid Agonists. Specifically, the risk for constipation and urinary retention may be increased with this combination. Monitor therapy
Oxatomide: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Potassium Chloride: Anticholinergic Agents may enhance the ulcerogenic effect of Potassium Chloride. Management: Patients on drugs with substantial anticholinergic effects should avoid using any solid oral dosage form of potassium chloride. Avoid combination
Potassium Citrate: Anticholinergic Agents may enhance the ulcerogenic effect of Potassium Citrate. Avoid combination
Pramlintide: May enhance the anticholinergic effect of Anticholinergic Agents. These effects are specific to the GI tract. Consider therapy modification
Ramosetron: Anticholinergic Agents may enhance the constipating effect of Ramosetron. Monitor therapy
Revefenacin: Anticholinergic Agents may enhance the anticholinergic effect of Revefenacin. Avoid combination
Secretin: Anticholinergic Agents may diminish the therapeutic effect of Secretin. Management: Avoid concomitant use of anticholinergic agents and secretin. Discontinue anticholinergic agents at least 5 half-lives prior to administration of secretin. Consider therapy modification
Thiazide and Thiazide-Like Diuretics: Anticholinergic Agents may increase the serum concentration of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Tiotropium: Anticholinergic Agents may enhance the anticholinergic effect of Tiotropium. Avoid combination
Topiramate: Anticholinergic Agents may enhance the adverse/toxic effect of Topiramate. Monitor therapy
Umeclidinium: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Adverse Reactions
Frequency not defined.
Cardiovascular: Flushing, palpitations, tachycardia
Central nervous system: Amnesia (short-term), ataxia, confusion (more common in elderly), dizziness, drowsiness, excitement (more common in elderly), fatigue, hallucination, headache, insomnia, nervousness, psychosis, speech disturbance
Dermatologic: Hypohidrosis, urticaria
Gastrointestinal: Abdominal pain, ageusia, bloating, constipation, diarrhea, dysgeusia, dysphagia, heartburn, nausea, vomiting, xerostomia
Genitourinary: Decreased lactation, impotence, urinary hesitancy, urinary retention
Hypersensitivity: Hypersensitivity reaction
Neuromuscular & skeletal: Weakness
Ophthalmic: Blurred vision, cycloplegia, increased intraocular pressure, mydriasis
Miscellaneous: Fever
Warnings/Precautions
Concerns related to adverse effect:
- CNS effects: May cause drowsiness and/or blurred vision, which may impair physical or mental abilities; patients must be cautioned about performing tasks which require mental alertness (eg, operating machinery or driving).
- Diarrhea: May be a sign of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy; treatment should be discontinued if this occurs.
- Heat prostration: May occur in the presence of increased environmental temperature; use caution in hot weather and/or exercise.
- Oral effects: Prolonged use may lead to development of dental caries, periodontal disease, oral candidiasis, or discomfort due to decreased salivation.
- Psychosis: Has been reported in patients with an extreme sensitivity to anticholinergic effects; usually resolves within 12-48 hours after discontinuation.
Disease-related concerns:
- Cardiovascular disease: Use with caution in patients with coronary artery disease, tachyarrhythmias, heart failure, or hypertension; evaluate tachycardia prior to administration. Use is contraindicated in patients with unstable cardiovascular status or myocardial ischemia.
- Hiatal hernia: Use with caution in patients with hiatal hernia with reflux esophagitis.
- Hyperthyroidism: Use with caution in patients with hyperthyroidism.
- Neuropathy: Use with caution in patients with autonomic neuropathy.
- Prostatic hyperplasia: Use with caution in patients with prostatic hyperplasia.
- Renal impairment: Use with caution in patients with renal impairment.
Special populations:
- Pediatric: Use with caution in children with spastic paralysis or brain damage; may be more susceptible to anticholinergic effects.
Dosage form specific issues:
- Benzyl alcohol and derivatives: Some dosage forms may contain sodium benzoate/benzoic acid; benzoic acid (benzoate) is a metabolite of benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC, 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol derivative with caution in neonates. See manufacturer’s labeling.
Pregnancy
Pregnancy Risk Factor
C
Pregnancy Considerations
Animal reproduction studies have not been conducted. Hyoscyamine crosses the placenta.
Patient Education
What is this drug used for?
- It is used to slow the speed in the stomach and GI (gastrointestinal) tract.
- It is used to treat infant belly pain.
- It is used to treat diarrhea.
- It is used to treat GI (gastrointestinal) ulcers.
- It is used to treat irritable bowel syndrome.
- It is used to treat muscle spasms of the GI (gastrointestinal) tract, gallbladder system, or urinary system.
- It is used to treat a runny nose.
- It is used to treat Parkinson disease.
- It is used to prevent irritation of the pancreas.
- It is used during surgery.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Fatigue
- Blurred vision
- Constipation
- Dry mouth
- Dry eyes
- Headache
- Nausea
- Vomiting
- Abdominal pain
- Loss of strength and energy
- Feeling full
- Change in taste
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Severe dizziness
- Passing out
- Severe diarrhea
- Confusion
- Mood changes
- Behavioral changes
- Sensing things that seem real but are not
- Trouble with memory
- Trouble sleeping
- Change in speech
- Change in balance
- Vision changes
- Eye pain
- Severe eye irritation
- Severe anxiety
- Difficult urination
- Lack of sweat
- Flushing
- Fast heartbeat
- Abnormal heartbeat
- Sexual dysfunction
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.