What is Imbruvica?
Imbruvica is a prescription medicine used to treat:
- Adults with mantle cell lymphoma (MCL) who have received at least one prior treatment.
- Adults with chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL).
- Adults with chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL) with 17p deletion.
- Adults with Waldenström’s macroglobulinemia (WM).
- Adults with marginal zone lymphoma (MZL) who require a medicine by mouth or injection (systemic therapy) and have received a certain type of prior treatment.
- Adults and children 1 year of age and older with chronic graft versus host disease (cGVHD) after failure of 1 or more lines of systemic therapy.
It is not known if Imbruvica is safe and effective in children under 1 year of age.
What should I tell my healthcare provider before taking Imbruvica?
Before taking Imbruvica, tell your healthcare provider about all of your medical conditions, including if you:
- have had recent surgery or plan to have surgery. Your healthcare provider may stop Imbruvica for any planned medical, surgical, or dental procedure.
- have bleeding problems
- have or had heart rhythm problems, smoke, or have a medical condition that increases your risk of heart disease, such as high blood pressure, high cholesterol, or diabetes
- have an infection
- have liver problems
- are pregnant or plan to become pregnant. Imbruvica can harm your unborn baby. If you are able to become pregnant, your healthcare provider will do a pregnancy test before starting treatment with Imbruvica. Tell your healthcare provider if you are pregnant or think you may be pregnant during treatment with Imbruvica.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with Imbruvica and for 1 month after the last dose.
- Males with female partners who are able to become pregnant should use effective birth control, such as condoms, during treatment with Imbruvica and for 1 month after the last dose.
- are breastfeeding or plan to breastfeed. Do not breastfeed during treatment with Imbruvica and for 1 week after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking Imbruvica with certain other medicines may affect how Imbruvica works and can cause side effects.
How should I take Imbruvica?
- Take Imbruvica exactly as your healthcare provider tells you to take it.
- Take Imbruvica 1 time a day at about the same time each day.
Imbruvica comes as capsules, tablets, and oral suspension.
- If your healthcare provider prescribes Imbruvica capsules or tablets:
- Swallow Imbruvica capsules or tablets whole with a glass of water.
- Do not open, break, or chew Imbruvica capsules.
- Do not cut, crush, or chew Imbruvica tablets.
- If your healthcare provider prescribes Imbruvica oral suspension:
- See the detailed Instructions for Use that comes with Imbruvica oral suspension for information about the correct way to give a dose to your child. If you have questions about how to give Imbruvica oral suspension, talk to your healthcare provider.
- Do not use if the carton seal is broken or missing.
- If you miss a dose of Imbruvica take it as soon as you remember on the same day. Take your next dose of Imbruvica at your regular time on the next day. Do not take extra doses of Imbruvica to make up for a missed dose.
- If you take too much Imbruvica call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Imbruvica?
You should not drink grapefruit juice, eat grapefruit, or eat Seville oranges (often used in marmalades) during treatment with Imbruvica. These products may increase the amount of Imbruvica in your blood.
What are the possible side effects of Imbruvica?
Imbruvica may cause serious side effects, including:
- Bleeding problems (hemorrhage) are common during treatment with Imbruvica, and can also be serious and may lead to death. Your risk of bleeding may increase if you are also taking a blood thinner medicine. Tell your healthcare provider if you have any signs of bleeding, including:
- blood in your stools or black stools (looks like tar)
- pink or brown urine
- unexpected bleeding, or bleeding that is severe or that you cannot control
- vomit blood or vomit looks like coffee grounds
- cough up blood or blood clots
- increased bruising
- dizziness
- weakness
- confusion
- change in your speech
- headache that lasts a long time or severe headache
- Infections can happen during treatment with Imbruvica. These infections can be serious and may lead to death. Tell your healthcare provider right away if you have fever, chills, weakness, confusion, or other signs or symptoms of an infection during treatment with Imbruvica.
- Heart problems. Serious heart rhythm problems (ventricular arrhythmias, atrial fibrillation and atrial flutter), heart failure and death have happened in people treated with Imbruvica, especially in people who have an infection, an increased risk for heart disease, or have had heart rhythm problems in the past. Your heart function will be checked before and during treatment with Imbruvica. Tell your healthcare provider if you get any symptoms of heart problems, such as feeling as if your heart is beating fast and irregular, lightheadedness, dizziness, shortness of breath, swelling of the feet, ankles or legs, chest discomfort, or you faint. If you develop any of these symptoms, your healthcare provider may do tests to check your heart and may change your Imbruvica dose.
- High blood pressure (hypertension). New or worsening high blood pressure has happened in people treated with Imbruvica. Your healthcare provider may start you on blood pressure medicine or change current medicines to treat your blood pressure.
- Decrease in blood cell counts. Decreased blood counts (white blood cells, platelets, and red blood cells) are common with Imbruvica, but can also be severe. Your healthcare provider should do monthly blood tests to check your blood counts.
- Second primary cancers. New cancers have happened during treatment with Imbruvica, including cancers of the skin or other organs.
- Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure, and sometimes death. Your healthcare provider may do blood tests to check you for TLS.
The most common side effects of Imbruvica in adults with B-cell malignancies (MCL, CLL/SLL, WM and MZL) include:
- diarrhea
- rash
- tiredness
- bruising
- muscle and bone pain
The most common side effects of Imbruvica in adults or children 1 year of age and older with cGVHD include:
- tiredness
- muscle and joint pain
- nausea
- low red blood cell count (anemia)
- fever
- stomach pain
- bruising
- muscle spasms
- pneumonia
- diarrhea
- mouth sores (stomatitis)
- headache
- low platelet count
- bleeding
Diarrhea is a common side effect in people who take Imbruvica. Drink plenty of fluids during treatment with Imbruvica to help reduce your risk of losing too much fluid (dehydration) due to diarrhea. Tell your healthcare provider if you have diarrhea that does not go away.
These are not all the possible side effects of Imbruvica.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Imbruvica Images
General information about the safe and effective use of Imbruvica
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Imbruvica for a condition for which it was not prescribed. Do not give Imbruvica to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Imbruvica that is written for health professionals.
How should I store Imbruvica?
- Store Imbruvica capsules and tablets at room temperature between 68°F and 77°F (20°C and 25°C).
- Keep Imbruvica capsules in the original container with the lid tightly closed.
- Keep Imbruvica tablets in the original carton.
- Store Imbruvica oral suspension bottle between 36°F and 77°F (2°C and 25°C). Do not freeze.
- Use Imbruvica oral suspension within 60 days after first opening the bottle. Throw away (discard) any unused portion 60 days after opening.
Keep Imbruvica and all medicines out of the reach of children.
What are the ingredients in Imbruvica?
Active ingredient: ibrutinib
Inactive ingredients:
Capsules: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate.
The 70 mg capsule shell contains gelatin, titanium dioxide, yellow iron oxide, and black ink.
The 140 mg capsule shell contains gelatin, titanium dioxide, and black ink.
Tablets: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate.
The film coating for each tablet contains ferrosoferric oxide (140 mg, 280 mg, and 420 mg tablets), polyvinyl alcohol, polyethylene glycol, red iron oxide (280 mg and 560 mg tablets), talc, titanium dioxide, and yellow iron oxide (140 mg, 420 mg, and 560 mg tablets).
Oral suspension: benzyl alcohol, citric acid monohydrate, disodium hydrogen phosphate, hypromellose, microcrystalline cellulose and carboxymethylcellulose sodium, purified water and sucralose.
For more information, go to www.imbruvica.com or call 1-877-877-3536.
Instructions for use for Imbruvica oral suspension
Imbruvica (im-BRU-vih-kuh)
(ibrutinib)
Oral Suspension, for oral use
This Instructions for Use contains information about how to prepare and give a dose of Imbruvica Oral Suspension.
Read this Instructions for Use before you give Imbruvica to your child, and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your child’s medical condition or treatment.
Call your healthcare provider or 1-877-877-3536 if you need help or have any questions about how to give Imbruvica the right way.
Important information you need to know before giving Imbruvica to your child.
- Imbruvica is for oral use only.
- Give Imbruvica exactly as your healthcare provider tells you to.
- If you miss giving a dose it can be given as soon as possible on the same day. Do not give more than the prescribed dose in 1 day.
 If your child takes too much Imbruvica call your healthcare provider for help.
If your child takes too much Imbruvica call your healthcare provider for help.
- Keep these instructions for future use.
Each Imbruvica carton contains (see Figure A):
- 1 bottle of Imbruvica (called ‘bottle’ in this Instructions for Use) with pre-inserted bottle adapter (called ‘adapter’ in this Instructions for Use). Do not remove the bottle adapter.
- 2 reusable 3 mL oral dosing syringes (called ‘syringe’ in this Instructions for Use) measuring in 0.1 mL increments.
 Only use the syringes that come with Imbruvica. Do not use the syringes for other patients or with other medicines.
Only use the syringes that come with Imbruvica. Do not use the syringes for other patients or with other medicines.
 If you cannot read the markings on the syringes, throw them away and call 1-877-877-3536 to get new ones.
If you cannot read the markings on the syringes, throw them away and call 1-877-877-3536 to get new ones.
Preparing and giving a dose of Imbruvica
Step 1: Gather and check supplies
- Check your child’s prescribed dose in milliliters (mLs). Find this mL marking on the syringe.
- If the dose is more than the marking on the syringe, split the dose between syringes as prescribed.
- Gather bottle and syringe(s) (see Figure A).
- Check the bottle and make sure that the bottle has Imbruvica Oral Suspension printed on it and the expiration date (“EXP”) has not passed.
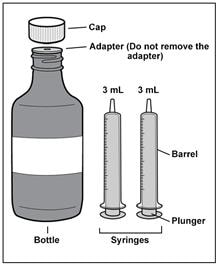
Figure A
 Do not use Imbruvica after the expiration date printed on the carton and the bottle after “EXP”.
Do not use Imbruvica after the expiration date printed on the carton and the bottle after “EXP”.
 Do not use if the Imbruvica carton seal appears to be tampered with.
Do not use if the Imbruvica carton seal appears to be tampered with.
Step 2: Record or check discard date
- When opening the bottle for the first time, record the date that is 60 days from the day the bottle is opened underneath the words “Discard Date” (see Figure B).
- Use Imbruvica within 60 days after opening.
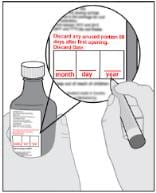
Figure B
 Do not use Imbruvica past the discard date recorded on the bottle.
Do not use Imbruvica past the discard date recorded on the bottle.
Step 3: Shake bottle
- Shake well before each use (see Figure C).
Figure C
Step 4: Remove cap from bottle
- Press down and twist the cap counterclockwise to remove it from the bottle (see Figure D).
- If there is fluid on top of the adapter you may wipe it with clean disposable tissue.
Do not remove the bottle adapter.
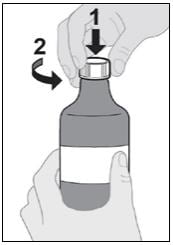
Figure D
Step 5: Attach syringe to bottle
- Make sure the syringe is clean and dry before use.
- Push the plunger down all the way.
- Gently insert tip of the syringe into the adapter.
- Turn the assembled bottle and syringe upside down (see Figure E).
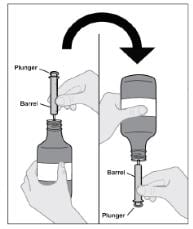
Figure E
Step 6: Fill syringe
- Slowly pull the syringe plunger down, past the number of mLs for your prescribed dose (see Figure F).
- Check for air bubbles and proceed to Step 7 for instructions on how to remove air bubbles.
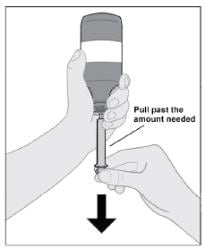
Figure F
Step 7: Remove air bubbles and adjust to the prescribed dose (mL)
- Hold the syringe and tap the sides to send bubbles to the tip.
- With the syringe attached to the bottle, push the plunger up to remove the air bubbles from the top (see Figure G).
- After the bubbles are removed, push the plunger up until the top of the colored plunger is even with the markings on the syringe for the dose.
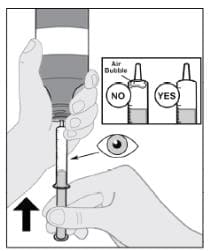
Figure G
 Air bubbles must be removed to ensure the correct dose.
Air bubbles must be removed to ensure the correct dose.
Note: Repeat steps 6 and 7 if any air bubbles remain.
Step 8: Remove syringe from bottle
- Turn the assembled bottle upright.
- Hold the middle of the syringe and carefully remove it from the bottle (see Figure H).
- Place the bottle aside.
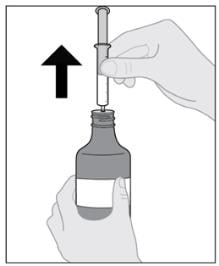
Figure H
 Do not touch the plunger of the syringe to avoid accidentally spilling the medicine before you are ready.
Do not touch the plunger of the syringe to avoid accidentally spilling the medicine before you are ready.
Note: If more than 1 syringe is needed to give the full dose, repeat steps 5 to 8 with the second syringe to complete the prescribed dose.
Step 9: Give Imbruvica
- Place the tip of the syringe along the inside of your child’s cheek.
- Slowly push the plunger all the way in to give the entire dose (see Figure I).
- Repeat with second syringe if needed to complete the prescribed dose.
Note: Imbruvica must be given as soon as possible after being drawn from the bottle.
Note: Make sure your child drinks water after swallowing the dose of medicine.
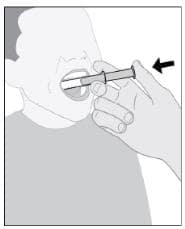
Figure I
Step 10: Recap bottle
- Place the cap back on the Imbruvica bottle (see Figure J).
- Make sure the bottle is tightly closed between each use.
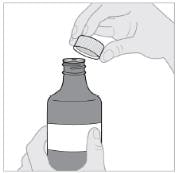
Figure J
Step 11: Rinse syringe
- Remove plunger from the syringe, rinse only with water and air dry (see Figure K).
- Store the syringe in a clean, dry place.
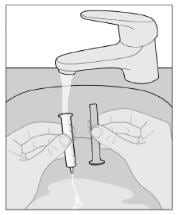
Figure K
 Do not clean the syringe with soap or in the dishwasher.
Do not clean the syringe with soap or in the dishwasher.
How to store Imbruvica Oral Suspension
- Store the bottle between 36°F and 77°F (2°C and 25°C).
 Do not freeze.
Do not freeze.
- Store Imbruvica and all medications out of sight and reach of children.
How to dispose of Imbruvica
 Dispose of (throw away) any unused medicine within 60 days after first opening of the bottle. At the same time throw away any used or unused syringes.
Dispose of (throw away) any unused medicine within 60 days after first opening of the bottle. At the same time throw away any used or unused syringes.
- Ask your pharmacist how to properly dispose of the medicine.
- For syringe disposal, rinse and place in household trash.
Instructions for use approved 8/2022.