What is Iyuzeh?
Iyuzeh is a prescription sterile eye drop solution which does not contain a preservative. Iyuzeh is used to lower the pressure in the eye (intraocular pressure) in people with open-angle glaucoma or ocular hypertension when their eye pressure is too high. Iyuzeh belongs to a group of medicines called prostaglandin analogs.
It is not known if Iyuzeh is safe and effective in children..
Who should not use Iyuzeh?
Do not use Iyuzeh if you are allergic latanoprost or any of the ingredients in Iyuzeh. See below for a complete list of ingredients in Iyuzeh.
What should I tell my healthcare provider before using Iyuzeh?
Before you use Iyuzeh, tell your doctor if you:
- have or have had eye problems including any surgery on your eye or eyes
- are using any other eye medicines
- have any other medical problems
- are pregnant or plan to become pregnant. It is not known if Iyuzeh will harm your unborn baby. If you become pregnant while using Iyuzeh talk to your doctor right away.
- are breastfeeding or plan to breastfeed. It is not known if Iyuzeh passes into your breast milk. Talk to your doctor about the best way to feed your baby if you use Iyuzeh.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use Iyuzeh?
Read the Instructions for Use at the end of this Patient Information leaflet for additional instructions about the right way to use Iyuzeh.
- Use 1 drop of Iyuzeh in your eye (or eyes) each evening. Talk to your doctor or pharmacist if you are not sure how to use Iyuzeh.
- Your Iyuzeh may not work as well if you use it more than 1 time each evening.
- If you miss a dose of Iyuzeh, skip the missed dose and take the next dose at your regular time.
- If you use other medicines in your eye, wait at least 5 minutes between using Iyuzeh and your other eye medicines.
- Contact lenses should be taken out before using Iyuzeh and you should wait at least 15 minutes after giving the dose of Iyuzeh before putting the contact lenses back into your eyes.
- Use your Iyuzeh right away after opening. Each Iyuzeh single-dose container is sterile and is to be used 1 time then thrown away. Do not save any Iyuzeh that may be left over after you use your medicine. Using Iyuzeh that is not sterile may cause other eye problems.
What are the possible side effects of Iyuzeh?
Iyuzeh may cause serious side effects including:
- changes in the color of your eye (iris). Your iris may become more brown in color while using Iyuzeh. This color change may not go away when you stop using Iyuzeh. If Iyuzeh is used in 1 eye only, the color of that eye may always be a different color from the color of your other eye.
- darkening of the color of the skin around your eye (eyelid). These skin changes usually go away when you stop using Iyuzeh.
- increasing the length, thickness, color, or number of your eyelashes. These eyelash changes usually go away when you stop using Iyuzeh.
- hair growth on your eyelids. This hair growth usually goes away when you stop using Iyuzeh.
The most common side effects of Iyuzeh include:
- redness of and around the eye (conjunctival hyperemia)
- abnormal sensation in the eye
- foreign body sensation in the eye
- eye irritation
- blurry vision
- eye itching
- increase of tears in the eye (increased lacrimation)
Tell your doctor right away if you have any new eye problems while using Iyuzeh, including:
- an eye injury
- an eye infection
- a sudden loss of vision
- eye surgery
- swelling and redness of and around your eye (conjunctivitis)
- problems with your eyelids
Additionally, the following side effects have been reported in other latanoprost medicines like Iyuzeh, when used in the eye (topical use):
- dizziness
- headache
- peeling skin over much of the body (toxic epidermal necrolysis)
- worsening of asthma
- shortness of breath (dyspnea)
- itching
- viral infection of the cornea (herpes keratitis)
- chest pain caused by interruptions in the heart’s blood supply, that can occur at rest (angina, unstable angina)
- awareness of heart rhythm (palpitations)
- chest pain
Tell your doctor if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of Iyuzeh. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Iyuzeh
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Iyuzeh for a condition for which it was not prescribed. Do not give Iyuzeh to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about Iyuzeh that is written for health professionals.
How should I store Iyuzeh?
Before opening the foil pouches:
- Store the unopened foil pouches between 59°F to 77°F (15°C to 25°C).
- Do not open the foil pouch containing Iyuzeh until you are ready to use the eye drops.
After opening the foil pouch:
- Store the opened foil pouch between 59°F to 77°F (15°C to 25°C), for up to 30 days.
- Write down the date of first opening the foil pouch in the space provided on the pouch.
- Throw away all unused Iyuzeh single-dose containers in the opened foil pouch after 30 days.
- Keep the Iyuzeh single-dose containers in their original foil pouch.
Keep Iyuzeh and all medicines out of the reach of children.
What are the ingredients in Iyuzeh?
Active ingredients: latanoprost
Inactive ingredients: polyoxyl 40 hydrogenated castor oil, sorbitol, carbomer 974P, polyethylene glycol 4000, disodium edetate, sodium hydroxide (for pH-adjustment) and water.
Instructions for use for Iyuzeh
Read these Instructions for Use before using your Iyuzeh and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
Important:
- Iyuzeh is for the eye. Do not swallow Iyuzeh.
- Iyuzeh single-dose containers are packaged in a foil pouch.
- Do not dose the Iyuzeh single-dose containers if the foil pouch is opened.
- Write down the date you open the foil pouch in the space provided on the pouch.
- Do not open the Iyuzeh single-dose container until you are ready to use the eye drops.
Please follow these instructions to use Iyuzeh:
Step 1. Wash your hands and sit or stand comfortably.
Step 2. Open the foil pouch containing a strip of 5 single-dose containers. Write down the date of first opening on the foil pouch.
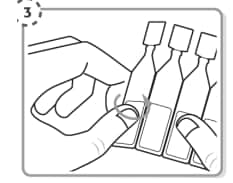
Step 3. Take the strip of single-dose containers from the foil pouch Break off one single-dose container from the strip Place the unopened single-dose containers back in the foil pouch and fold the edge to close the pouch.
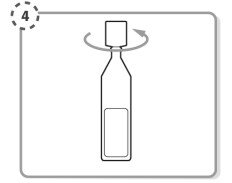
Step 4. Hold the single-dose container upright. Make sure that your Iyuzeh medicine is in the bottom part of the single-dose container. Twist open the top of the single-dose container as shown. Do not touch the tip after opening the container.
Step 5. Tilt your head backwards. If you are unable to tilt your head, lie down. Use your finger to gently pull down the lower eyelid of your affected eye.
Step 6. Place the tip of the single-dose container close to, but not touching your eye.
Step 7. Squeeze the single-dose container gently so that only one drop goes into your eye, then release the lower eyelid. If the drop misses your eye completely, try again.
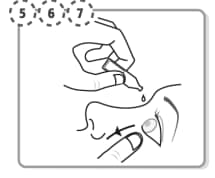
- If your doctor has told you to use Iyuzeh drops in both eyes, repeat Steps 5 to Step 7 for your other eye.
- Each single-dose container contains enough solution for both eyes.
- Throw away the single-dose container after use. Do not keep it to use it again. To lessen the chance of an infection, a new single-dose container must be opened each time you are ready to use Iyuzeh.
- Place the unopened foil pouch with the unopened single-dose containers back in the carton. The unopened single-dose containers must be used within 30 days after opening the foil pouch.
Instructions for use approved 12/2022




