Boxed Warning
Hematologic toxicity:
Zidovudine, a component of lamivudine/zidovudine tablets, has been associated with hematologic toxicity, including neutropenia and severe anemia, particularly in patients with advanced HIV-1 disease.
Myopathy:
Prolonged use of zidovudine has been associated with symptomatic myopathy.
Exacerbations of hepatitis B:
Severe, acute exacerbations of hepatitis B have been reported in patients who are coinfected with hepatitis B virus (HBV) and HIV-1 and have discontinued lamivudine, a component of lamivudine/zidovudine. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue lamivudine/zidovudine and are coinfected with HIV-1 and HBV. If appropriate, initiation of antihepatitis B therapy may be warranted.
Lactic acidosis and severe hepatomegaly with steatosis:
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with use of nucleoside analogues, including lamivudine and zidovudine. Discontinue lamivudine/zidovudine if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
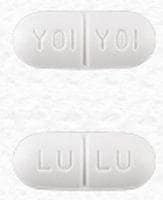
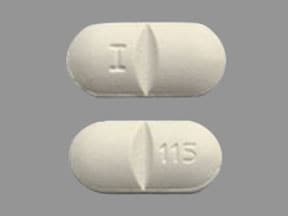
Combivir: Lamivudine 150 mg and zidovudine 300 mg [scored]
Generic: Lamivudine 150 mg and zidovudine 300 mg
Pharmacology
Mechanism of Action
The combination of zidovudine and lamivudine is believed to act synergistically to inhibit reverse transcriptase via DNA chain termination after incorporation of the nucleoside analogue as well as to delay the emergence of mutations conferring resistance
Use: Labeled Indications
HIV-1 infection: Treatment of HIV-1 infection in combination with other antiretrovirals.
Contraindications
Hypersensitivity to lamivudine or zidovudine, or any component of the formulation.
Canadian labeling: Additional contraindications (not in US labeling): Neutrophil count <750/mm3 or hemoglobin <7.5 g/dL (4.65 mmol/L)
Dosage and Administration
Dosing: Adult
Note: Because this is a fixed-dose combination product, avoid use in patients requiring dosage reduction.
HIV-1 infection, treatment: Oral: One tablet twice daily.
Dosing: Pediatric
Note: Use in combination with at least one other antiretroviral agent.
HIV-1 Treatment:
Children and Adolescents weighing <30 kg: Not intended for use; product is a fixed-dose combination; safety and efficacy have not been established in these patients
Children and Adolescents weighing ≥30 kg: Oral: One tablet twice daily
Administration
Oral: Administer without regard to food.
Storage
Store between 2°C and 30°C (36°F and 86°F).
Lamivudine and Zidovudine Images
Drug Interactions
Acemetacin: May enhance the adverse/toxic effect of Zidovudine. Specifically, the risk for hematologic toxicity may be increased. Monitor therapy
Acyclovir-Valacyclovir: May enhance the CNS depressant effect of Zidovudine. Monitor therapy
Amodiaquine: Zidovudine may enhance the neutropenic effect of Amodiaquine. Avoid combination
BCG (Intravesical): Myelosuppressive Agents may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Cabozantinib: MRP2 Inhibitors may increase the serum concentration of Cabozantinib. Monitor therapy
Chloramphenicol (Ophthalmic): May enhance the adverse/toxic effect of Myelosuppressive Agents. Monitor therapy
Cladribine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Avoid combination
Cladribine: Agents that Undergo Intracellular Phosphorylation may diminish the therapeutic effect of Cladribine. Avoid combination
Clarithromycin: May enhance the myelosuppressive effect of Zidovudine. Clarithromycin may decrease the serum concentration of Zidovudine. Management: Monitor response to zidovudine closely when used with clarithromycin, and consider staggering zidovudine and clarithromycin doses when possible in order to minimize the potential for interaction. Consider therapy modification
CloZAPine: Myelosuppressive Agents may enhance the adverse/toxic effect of CloZAPine. Specifically, the risk for neutropenia may be increased. Monitor therapy
Deferiprone: Myelosuppressive Agents may enhance the neutropenic effect of Deferiprone. Management: Avoid the concomitant use of deferiprone and myelosuppressive agents whenever possible. If this combination cannot be avoided, monitor the absolute neutrophil count more closely. Consider therapy modification
Dexketoprofen: May enhance the adverse/toxic effect of Zidovudine. Monitor therapy
Dipyrone: May enhance the adverse/toxic effect of Myelosuppressive Agents. Specifically, the risk for agranulocytosis and pancytopenia may be increased Avoid combination
DOXOrubicin (Conventional): May enhance the adverse/toxic effect of Zidovudine. DOXOrubicin (Conventional) may diminish the therapeutic effect of Zidovudine. Consider therapy modification
DOXOrubicin (Liposomal): May enhance the adverse/toxic effect of Zidovudine. DOXOrubicin (Liposomal) may diminish the therapeutic effect of Zidovudine. Consider therapy modification
Emtricitabine: LamiVUDine may enhance the adverse/toxic effect of Emtricitabine. Avoid combination
Fluconazole: May decrease the metabolism of Zidovudine. Monitor therapy
Ganciclovir-Valganciclovir: May enhance the adverse/toxic effect of Zidovudine. Specifically, hematologic toxicity may be enhanced. Monitor therapy
Interferons: May enhance the adverse/toxic effect of Zidovudine. Interferons may decrease the metabolism of Zidovudine. Monitor therapy
Levomethadone: May increase the serum concentration of Zidovudine. Monitor therapy
Mesalamine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Methadone: May increase the serum concentration of Zidovudine. Monitor therapy
Nitisinone: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Orlistat: May decrease the serum concentration of Antiretroviral Agents. Monitor therapy
Pretomanid: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Probenecid: May decrease the metabolism of Zidovudine. Monitor therapy
Promazine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Protease Inhibitors: May decrease the serum concentration of Zidovudine. Monitor therapy
Raltegravir: May enhance the myopathic (rhabdomyolysis) effect of Zidovudine. Monitor therapy
Ribavirin (Oral Inhalation): Zidovudine may enhance the adverse/toxic effect of Ribavirin (Oral Inhalation). Specifically, the risk/severity of anemia may be increased. Management: Due to significantly increased risk of anemia, consider even closer monitoring for anemia than routinely recommended. Alternative therapies should be considered when clinically possible, particularly for patients with other risk factors. Consider therapy modification
Ribavirin (Systemic): Zidovudine may enhance the adverse/toxic effect of Ribavirin (Systemic). Specifically, the risk/severity of anemia may be increased. Management: Due to significantly increased risk of anemia, consider even closer monitoring for anemia than routinely recommended for ribavirin. Alternative therapies should be considered when clinically possible, particularly for patients with other risk factors. Consider therapy modification
Rifamycin Derivatives: May decrease the serum concentration of Zidovudine. Exceptions: Rifabutin. Monitor therapy
Sorbitol: May decrease the serum concentration of LamiVUDine. Management: When possible, avoid chronic coadministration of sorbitol-containing solutions with lamivudine, but if this combination cannot be avoided, monitor patients more closely for possible therapeutic failure associated with decreased lamivudine exposure. Consider therapy modification
Stavudine: Zidovudine may diminish the therapeutic effect of Stavudine. Avoid combination
Tenoxicam: May enhance the adverse/toxic effect of Zidovudine. Monitor therapy
Teriflunomide: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Tolvaptan: May increase the serum concentration of OAT1/3 Substrates. Management: Patients being treated with the Jynarque brand of tolvaptan should avoid concomitant use of OAT1/3 substrates. Concentrations and effects of the OAT1/3 substrate would be expected to increase with any combined use. Consider therapy modification
Trimethoprim: May increase the serum concentration of LamiVUDine. Monitor therapy
Valproate Products: May increase the serum concentration of Zidovudine. Monitor therapy
Adverse Reactions
See individual agents.
Warnings/Precautions
Concerns related to adverse effects:
- Hematologic toxicity: [US Boxed Warning]: Zidovudine is associated with hematologic toxicity, including neutropenia and severe anemia. Use with caution in patients with bone marrow compromise (granulocytes <1,000 cells/mm3 or hemoglobin <9.5 g/dL).
- Immune reconstitution syndrome: Patients may develop immune reconstitution syndrome resulting in the occurrence of an inflammatory response to an indolent or residual opportunistic infection during initial HIV treatment or activation of autoimmune disorders (eg, Graves disease, polymyositis, Guillain-Barré syndrome) later in therapy; further evaluation and treatment may be required.
- Lactic acidosis/hepatomegaly: [US Boxed Warning]: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues and other antiretrovirals. Female gender and obesity may increase the risk for development. Suspend treatment in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or hepatotoxicity (transaminase elevation may/may not accompany hepatomegaly and steatosis).
- Lipoatrophy: Zidovudine may cause loss of subcutaneous fat, especially in the face, limbs, and buttocks. Lipoatrophy incidence and severity are related to cumulative exposure and may be only partially reversible; improvement may take months to years after switching to a regimen that does not contain zidovudine. Monitor patients for signs of lipoatrophy and consider switching to a non-zidovudine-containing regimen if lipoatrophy occurs.
- Myopathy: [US Boxed Warning]: Prolonged use of zidovudine has been associated with symptomatic myopathy.
Disease-related concerns:
- Chronic hepatitis B: [US Boxed Warning]: Severe acute exacerbations of hepatitis B have been reported in patients coinfected with HBV and HIV-1 when therapy is discontinued; monitor patients with clinical and laboratory follow-up for at least several months after treatment discontinuation. Emergence of hepatitis B virus lamivudine-resistant variants has been reported in patients with concurrent HBV infection who received a lamivudine-containing regimen for HIV-1 treatment.
- Hepatic impairment: Use is not recommended in patients with hepatic impairment.
- Pancreatitis: Use with caution in patients with a history of pancreatitis or other significant risk factors for pancreatitis development. Discontinue immediately if clinical signs, symptoms, or laboratory abnormalities suggestive of pancreatitis occur.
- Renal impairment: Use is not recommended in patients with renal impairment (CrCl <50 mL/minute).
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Monitoring Parameters
Amylase, bilirubin, signs and symptoms of pancreatitis. Monitor CBC with differential and platelet count at least every 2 weeks, liver function tests (including signs/symptoms of hepatomegaly), MCV, serum creatinine kinase, viral load, and CD4 count; observe for appearance of opportunistic infections; signs of muscle weakness or pain; blood lactate levels and signs of acidosis
Pregnancy
Pregnancy Considerations
The Health and Human Services (HHS) Perinatal HIV Guidelines consider lamivudine in combination with zidovudine an alternative NRTI backbone for pregnant females who are antiretroviral-naive, females who have had antiretroviral therapy (ART) in the past but are restarting, who require a new ART regimen (due to poor tolerance or poor virologic response of current regimen), and who are not yet pregnant but are trying to conceive. Females who become pregnant while taking this combination may continue if viral suppression is effective and the regimen is well tolerated. Although use of this combination has the most experience for use in pregnancy, it has an increased potential for hematologic toxicity and requires twice-daily dosing (HHS [perinatal] 2019).
Refer to individual monographs for additional information.
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience headache, nausea, vomiting, diarrhea, stuffy nose, runny nose, lack of appetite, dizziness, abdominal pain, abdominal cramps, joint pain, or trouble sleeping. Have patient report immediately to prescriber signs of infection, signs of lactic acidosis (fast breathing, fast heartbeat, abnormal heartbeat, vomiting, fatigue, shortness of breath, severe loss of strength and energy, severe dizziness, feeling cold, or muscle pain or cramps), signs of liver problems (dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin), signs of pancreatitis (severe abdominal pain, severe back pain, severe nausea, or vomiting), depression, burning or numbness feeling, severe loss of strength and energy, muscle pain, muscle weakness, or change in body fat (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer:Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.