What is Lantus?
Lantus is a long-acting man-made insulin used to control high blood sugar in adults and children with diabetes mellitus. Lantus is not for use to treat diabetic ketoacidosis.
What is the most important information I should know about Lantus?
Do not share your syringes with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
Do not share your Lantus SoloStar pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
Who should not use Lantus?
Do not use Lantus if you:
- are having an episode of low blood sugar (hypoglycemia).
- have an allergy to insulin glargine or any of the ingredients in Lantus. See below Information guide for a complete list of ingredients in Lantus.
What should I tell my healthcare provider before using Lantus?
Before using Lantus, tell your healthcare provider about all your medical conditions including if you:
- have liver or kidney problems.
- take other medicines, especially ones called TZDs (thiazolidinediones).
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take TZDs with Lantus.
- are pregnant, planning to become pregnant, or are breastfeeding. It is not known if Lantus may harm your unborn baby or breastfeeding baby.
Tell your healthcare provider about all the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Before you start using Lantus, talk to your healthcare provider about low blood sugar and how to manage it.
How should I use Lantus?
- Read the detailed Instructions for Use that come with your Lantus insulin or your Lantus SoloStar single-patient-use pen.
- Use Lantus exactly as your healthcare provider tells you to. Your healthcare provider should tell you how much Lantus to use and when to use it.
- Know the amount of Lantus you use. Do not change the amount of Lantus you use unless your healthcare provider tells you to.
- Check your insulin label each time you give your injection to make sure you are using the correct insulin.
- The dose counter on your SoloStar pen shows your dose of Lantus. Do not make any dose changes unless your healthcare provider tells you to.
- Do not use a syringe to remove Lantus from your SoloStar disposable prefilled pen.
- Do not re-use needles. Always use a new needle for each injection. Re-use of needles increases your risk of having blocked needles, which may cause you to get the wrong dose of Lantus. Using a new needle for each injection lowers your risk of getting an infection.
- You may take Lantus at any time during the day but you must take it at the same time every day.
- Only use Lantus that is clear and colorless. If your Lantus is cloudy or slightly colored, return it to your pharmacy for a replacement.
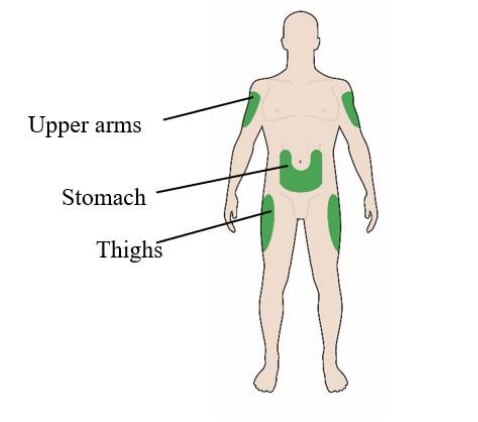
- Lantus is injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- Do not use Lantus in an insulin pump or inject Lantus into your vein (intravenously).
- Change (rotate) injection sites within the area you chose with each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not mix Lantus with any other type of insulin or liquid medicine.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugar should be and when you should check your blood sugar levels.
Keep Lantus and all medicines out of the reach of children.
Your dose of Lantus may need to change because of:
- a change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of the medicines you take.
What should I avoid while taking Lantus?
While using Lantus do not:
- drive or operate heavy machinery, until you know how Lantus affects you.
- drink alcohol or use over-the-counter medicines that contain alcohol.
What are the possible side effects of Lantus and other insulins?
Lantus may cause serious side effects that can lead to death, including:
- Low blood sugar (hypoglycemia). Signs and symptoms that may indicate low blood sugar include:
- dizziness or light-headedness, sweating, confusion, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability or mood change, hunger.
- Severe allergic reaction (whole body reaction). Get medical help right away if you have any of these signs or symptoms of a severe allergic reaction:
- a rash over your whole body, trouble breathing, a fast heartbeat, or sweating
- Low potassium in your blood (hypokalemia).
- Heart failure. Taking certain diabetes pills called TZDs (thiazolidinediones) with Lantus may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with Lantus. Your healthcare provider should monitor you closely while you are taking TZDs with Lantus. Tell your healthcare provider if you have any new or worse symptoms of heart failure including:
- shortness of breath, swelling of your ankles or feet, sudden weight gain.
Treatment with TZDs and Lantus may need to be changed or stopped by your healthcare provider if you have new or worse heart failure.
Get emergency medical help if you have:
- trouble breathing; shortness of breath; fast heartbeat; swelling of your face, tongue, or throat; sweating; extreme drowsiness; dizziness; confusion.
The most common side effects of Lantus include:
- low blood sugar (hypoglycemia), weight gain, allergic reactions, including reactions at your injection site, skin thickening or pits at the injection site (lipodystrophy)
These are not all the possible side effects of Lantus. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Drug Interactions
A total of 392 medications are known to interact with Lantus and Lantus SoloStar. Use the Interactions Checker Tool.
Common Interactions Checks
General information about the safe and effective use of Lantus
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Lantus for a condition for which it was not prescribed. Do not give Lantus to other people, even if they have the same symptoms that you have. It may harm them.
This guide summarizes the most important information about Lantus. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Lantus that is written for healthcare professionals. For more information about Lantus call 1-800-633-1610 or go to the website www.lantus.com.
How should I store Lantus?
Unopened (not in-use) Lantus vials
- Store unused Lantus vials in the refrigerator from 36°F to 46°F (2°C to 8°C).
- Do not freeze Lantus.
- Keep Lantus away from direct heat and light.
- If a vial has been frozen or overheated, throw it away.
- Unopened vials can be used until the expiration date on the carton and vial label if they have been stored in the refrigerator (they can be stored past 28 days in the refrigerator). •
- Unopened vials should be thrown away after 28 days if they are stored at room temperature.
After Lantus vials have been opened (in-use)
- Store in-use (opened) Lantus vials in a refrigerator from 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C) for up to 28 days.
- Do not freeze Lantus. If a vial has been frozen, throw it away.
- Keep Lantus out of direct heat and light.
- The Lantus vial you are using should be thrown away after 28 days or if the expiration date has passed, even if it still has insulin left in it.
Unused (not-in-use) Lantus Solostar Pen
- Before first use keep new pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze
- Do not use Lantus if it has been frozen.
After first use of Lantus Solostar Pen
- Keep your pen at room temperature below 86°F (30°C).
- Keep your pen away from heat or light.
- Store your pen with the pen cap on.
- Do not put your pen back in the refrigerator.
- Do not store your pen with the needle attached.
- Keep out of the reach of children.
- Only use your pen for up to 28 days after its first use. Throw away the Lantus SoloStar pen you are using after 28 days, even if it still has insulin left in it.
Caring for Your Lantus SoloStar Pen
Handle your pen with care
- Do not drop your pen or knock it against hard surfaces.
- If you think that your pen may be damaged, do not try to fix it. Use a new one.
Protect your pen from dust and dirt
- You can clean the outside of your pen by wiping it with a damp cloth (water only). Do not soak, wash or lubricate your pen. This may damage it.
Disposing Lantus SoloStar Pen
- The used Lantus SoloStar pen may be thrown away in your household trash after you have removed the needle. •
- Put the used needle in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the used needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
What are the ingredients in Lantus?
Active ingredient: insulin glargine
Inactive ingredients:
10 mL vial: glycerol 85%, m-cresol, polysorbate 20, zinc, and Water for Injection, USP. Hydrochloric acid and sodium hydroxide may be added to adjust the pH.
3 mL SoloStar prefilled pen: glycerol 85%, m-cresol, zinc, and Water for Injection, USP. Hydrochloric acid and sodium hydroxide may be added to adjust the pH.
Instructions for use for Lantus
Lantus SOLOSTAR (LAN-tus)
(insulin glargine)
injection, for subcutaneous use
3 mL Single-Patient-Use Prefilled Pen: 100 Units/mL (U-100)
Read these Instructions for Use before you start taking the Lantus Solostar pen and each time you get a new Lantus SoloStar pen. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your Lantus SoloStar pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
People who are blind or have vision problems should not use the Lantus SoloStar prefilled pen without help from a person trained to use the Lantus SoloStar prefilled pen.
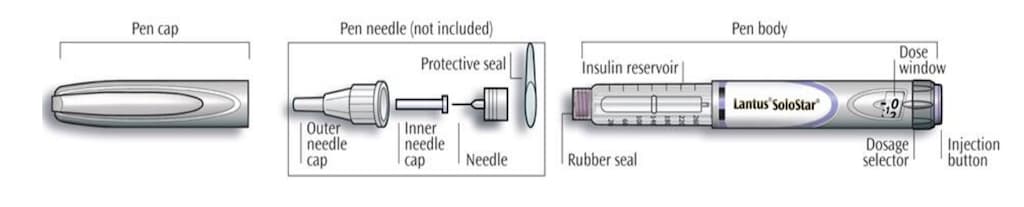
Lantus SoloStar is a disposable prefilled pen used to inject Lantus. Each Lantus SoloStar pen has 300 units of insulin which can be used for multiple injections. You can select doses from 1 to 80 units in steps of 1 unit. The pen plunger moves with each dose. The plunger will only move to the end of the cartridge when 300 units of Lantus have been given.
Important Information You Need to Know Before Injecting Lantus
- Do not use your pen if it is damaged or if you are not sure that it is working properly.
- Do not use a syringe to remove Lantus from your pen.
- Do not reuse needles. If you do, you might get the wrong dose of Lantus and/or increase the chance of getting an infection.
- Always perform a safety test (see Step 3).
- Always carry a spare pen and spare needles in case they got lost or stop working.
- Change (rotate) your injection sites within the area you choose for each dose (see “Places to inject”).
Learn to Inject
- Talk with your healthcare provider about how to inject before using your pen.
- Ask for help if you have problems handling the pen, for example if you have problems with your sight.
- Read all these instructions before using your pen. If you do not follow all these instructions, you may get too much or too little insulin. Need Help? If you have any questions about your pen or about diabetes, ask your healthcare provider, or go to www.Lantus.com or call sanofi-aventis at 1-800-633-1610.
Extra Items You Will Need
- a new sterile needle (see Step 2).
- an alcohol swab.
- a puncture-resistant container for used needles and pens. (See “Throwing your pen away”)
Places to Inject
- Inject your insulin exactly as your healthcare provider has shown you.
- Inject your insulin under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin
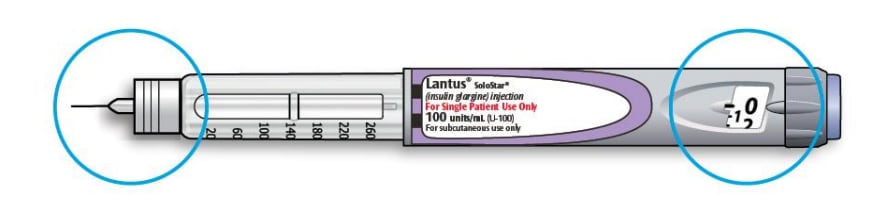
Get to know your pen
Step 1: Check your pen
Take a new pen out of the refrigerator at least 1 hour before you inject. Cold insulin is more painful to inject.
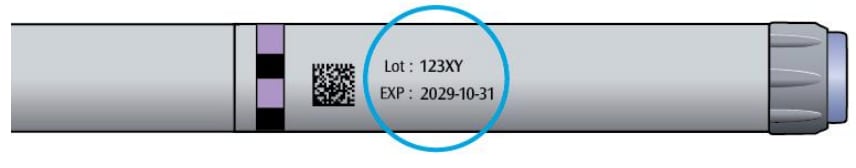
1A Check the name and expiration date on the label of your pen.
- Make sure you have the correct insulin.
- Do not use your pen after the expiration date.
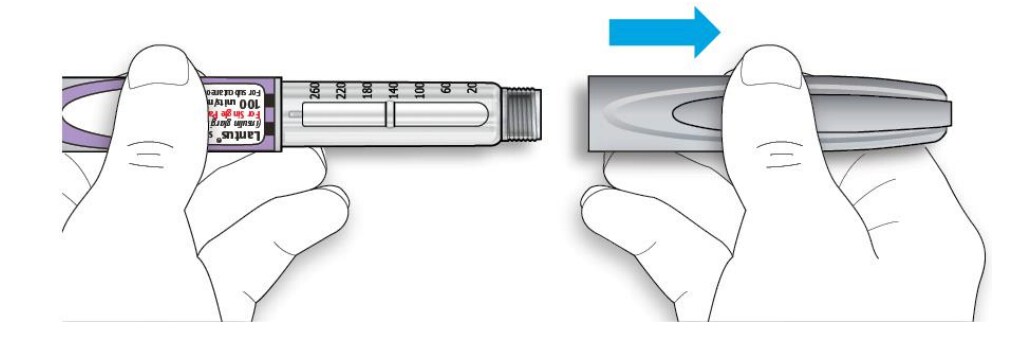
1B Pull off the pen cap.
1C Check that the insulin is clear.
- Do not use the pen if the insulin looks cloudy, colored or contains particles.
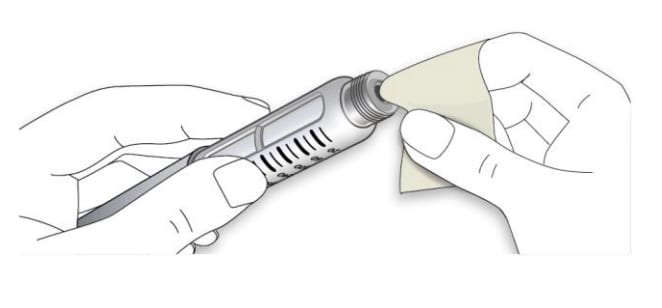
1D Wipe the rubber seal with an alcohol swab.
If you have other injector pens:
- Making sure you have the correct medicine is especially important if you have other injector pens.
Step 2: Attach a new needle
- Do not reuse needles. Always use a new sterile needle for each injection. This helps stop blocked needles, contamination, and infection.
Only use needles* that are compatible for use with Lantus SoloStar, such as BD UltraFine
2A Take a new needle and peel off the protective seal.
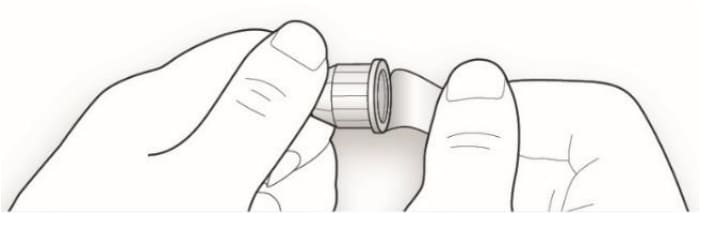
2B Keep the needle straight and screw it onto the pen until fixed. Do not over-tighten.
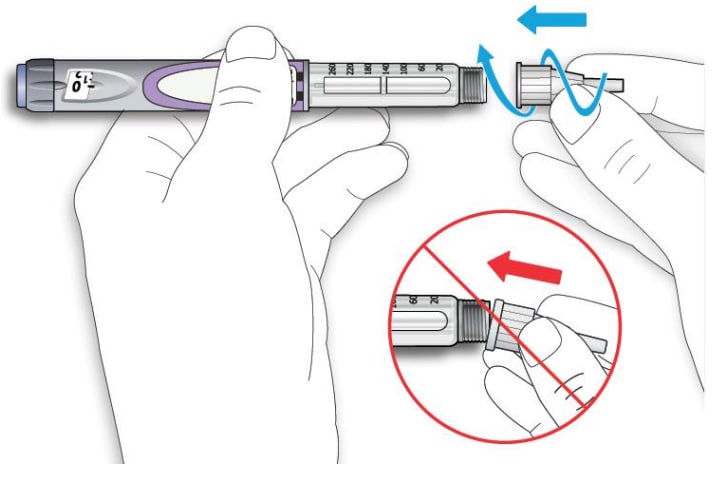
2C Pull off the outer needle cap. Keep this for later.
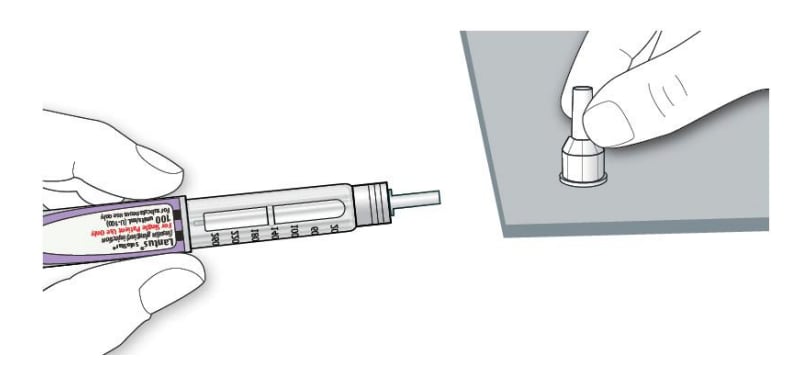
2D Pull off the inner needle cap and throw away.
Handling needles
- Take care when handling needles to prevent needle-stick injury and cross-infection.
Step 3: Do a safety test
Always do a safety test before each injection to:
- Check your pen and the needle to make sure they are working properly. •
- Make sure that you get the correct Lantus dose.
3A Select 2 units by turning the dose selector until the dose pointer is at the 2 mark.
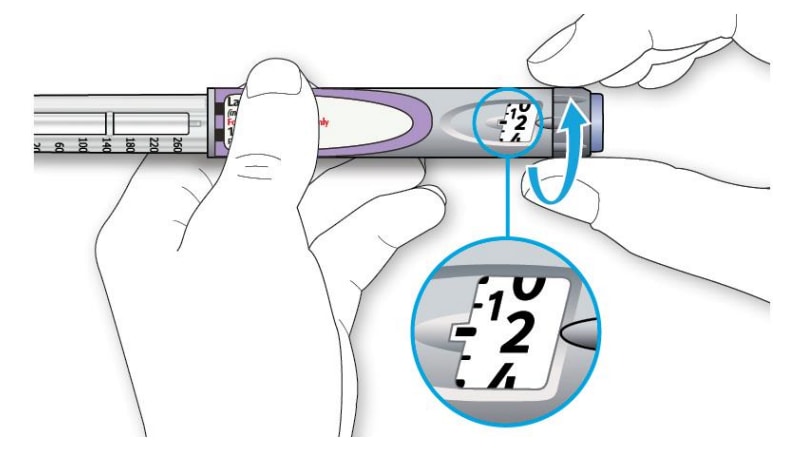
3B Press the injection button all the way in.
When insulin comes out of the needle tip, your pen is working correctly:
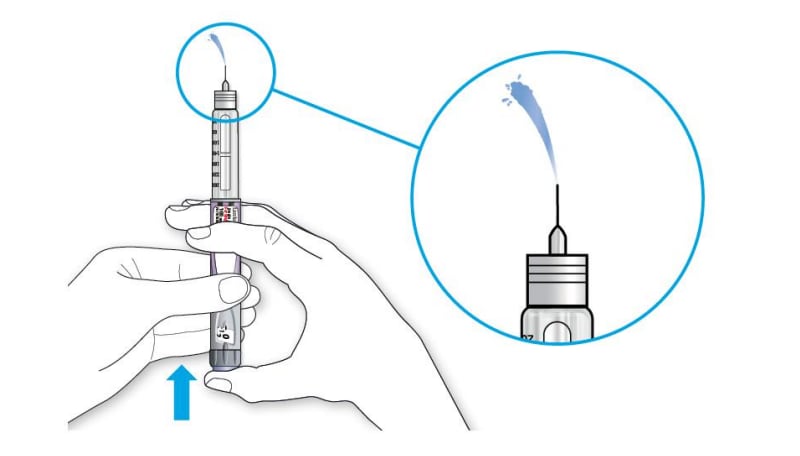
If no insulin appears:
- You may need to repeat this step up to 3 times before seeing insulin.
- If no insulin comes out after the third time, the needle may be blocked. If this happens:
- change the needle (see Step 6 and Step 2),
- then repeat the safety test (Step 3).
- Do not use your pen if there is still no insulin coming out of the needle tip. Use a new pen.
- Do not use a syringe to remove insulin from your pen.
If you see air bubbles:
- You may see air bubbles in the insulin. This is normal, they will not harm you.
Step 4: Select the dose
Do not select a dose or press the injection button without a needle attached. This may damage your pen.
4A Make sure a needle is attached and the dose is set to “0.”
4B Turn the dose selector until the dose pointer lines up with your dose.
- If you turn past your dose, you can turn back down.
- If there are not enough units left in your pen for your dose, the dose selector will stop at the number of units left.
- If you cannot select your full prescribed dose, use a new pen or inject the remaining units and use a new pen to complete your dose.
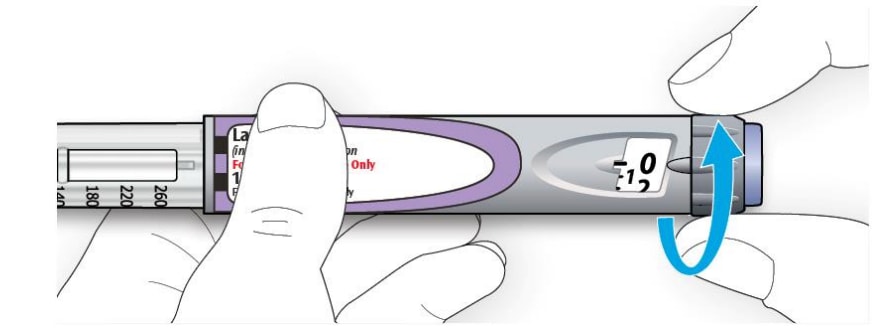
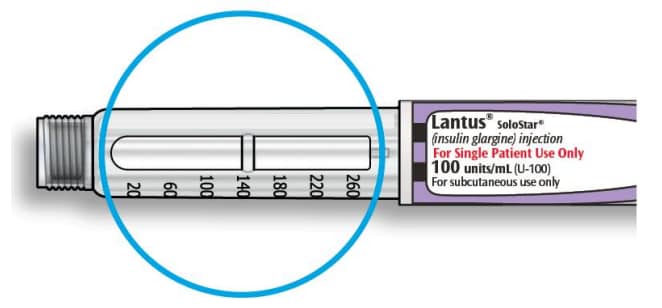
How to read the dose window
Even numbers are shown in line with dose pointer.
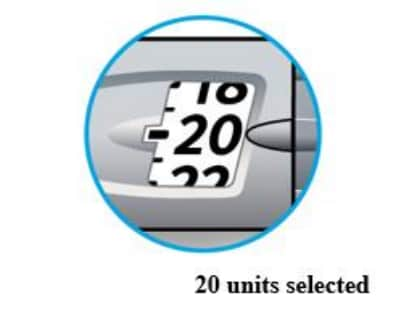
Odd numbers are shown as a line between even numbers.
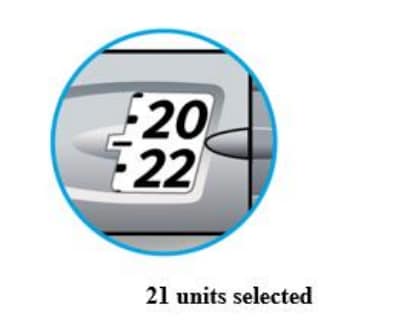
Units of Lantus in your pen:
- Your pen contains a total of 300 units of Lantus. You can select doses from 1 to 80 units in steps of 1 unit. Each pen contains more than 1 dose.
- You can see roughly how many units of insulin are left by looking at where the plunger is on the insulin scale.
Step 5: Injecting Your Lantus Dose
If you find it hard to press the injection button in, do not force it as this may break your pen. See the section below for help.
5A Choose a place to inject as shown in the picture above.
5B Push the needle into your skin as shown by your healthcare provider.
Do not touch the injection button yet.
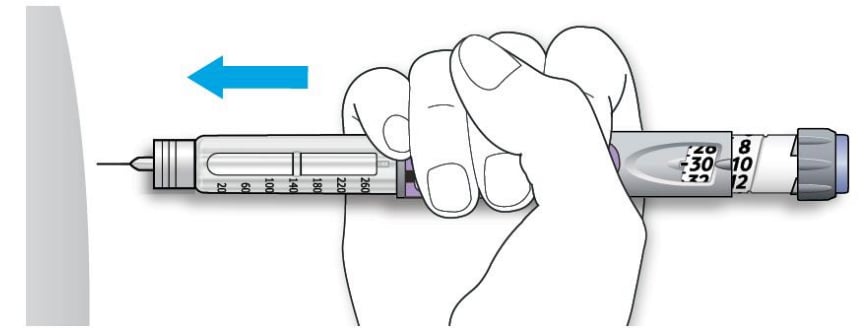
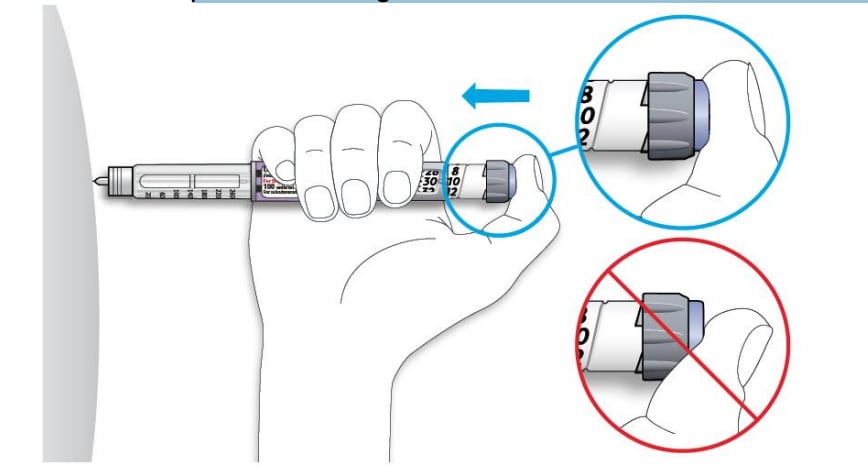
5C Place your thumb on the injection button. Then press all the way in and hold.
- Do not press at an angle. Your thumb could block the dose selector from turning.
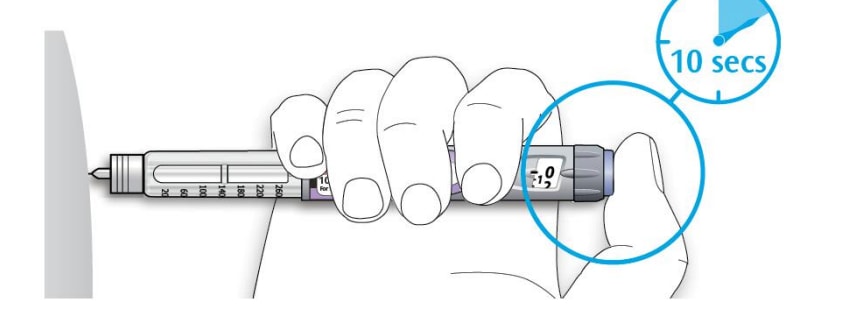
5D Keep the injection button held in and when you see "0" in the dose window, slowly count to 10.
- This will make sure you get your full dose.
5E After holding and slowly counting to 10, release the injection button. Then remove the needle from your skin.
If you find it hard to press the button in:
- Change the needle (see Step 6 and Step 2) then do a safety test (see Step 3).
- If you still find it hard to press in, get a new pen.
- Do not use a syringe to remove insulin from your pen.
Step 6: Remove the needle
- Take care when handling needles to prevent needle-stick injury and cross-infection.
- Do not put the inner needle cap back on.
6A Grip the widest part of the outer needle cap. Keep the needle straight and guide it into the outer needle cap. Then push firmly on.
- The needle can puncture the cap if it is recapped at an angle.
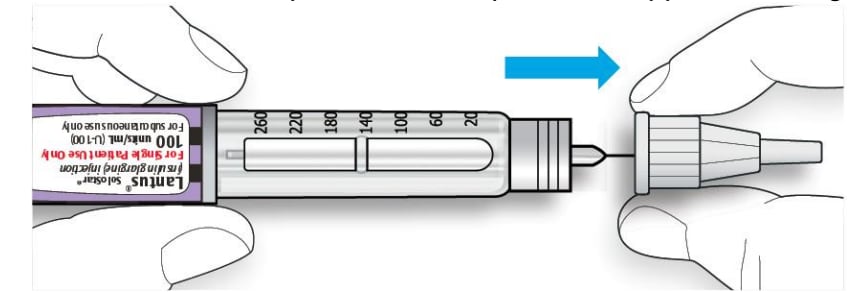
6B Grip and squeeze the widest part of the outer needle cap. Turn your pen several times with your other hand to remove the needle.
- Try again if the needle does not come off the first time
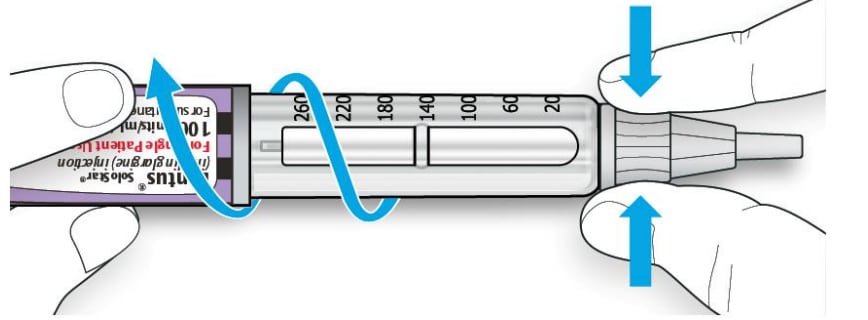
6C Throw away the used needle in a puncture-resistant container (see “Throwing your pen away” at the end of this Instructions for Use).
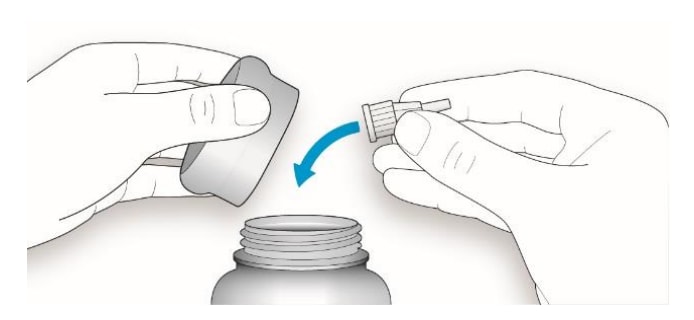
6D Put your pen cap back on.
- Do not put the pen back in the refrigerator
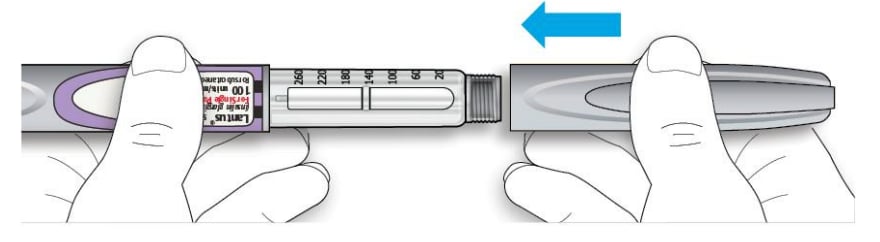
Storing the Lantus Solostar Pen
Before first use
- Keep new pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze. Do not use Lantus if it has been frozen.
After first use
- Keep your pen at room temperature below 86°F (30°C).
- Keep your pen away from heat or light.
- Store your pen with the pen cap on.
- Do not put your pen back in the refrigerator.
- Do not store your pen with the needle attached.
- Keep out of the reach of children.
- Only use your pen for up to 28 days after its first use. Throw away the Lantus SoloStar pen you are using after 28 days, even if it still has insulin left in it.
Caring for Your Lantus SoloStar Pen
Handle your pen with care
- Do not drop your pen or knock it against hard surfaces.
- If you think that your pen may be damaged, do not try to fix it. Use a new one.
Protect your pen from dust and dirt
- You can clean the outside of your pen by wiping it with a damp cloth (water only). Do not soak, wash or lubricate your pen. This may damage it.
Disposing Lantus SoloStar Pen
- The used Lantus SoloStar pen may be thrown away in your household trash after you have removed the needle.
- Put the used needle in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the used needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Instructions for use revised 06/2022.
Lantus (LAN-tus)
(insulin glargine)
injection, for subcutaneous use
10 mL Vial (100 Units/mL, U-100)
These Instructions for Use contain information on how to inject Lantus using the vial. Read these Instructions for Use before you start taking Lantus and each time you get a new Lantus vial. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your Lantus syringes with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
Supplies needed to give your injection:
- a Lantus 10 mL vial
- a U-100 insulin syringe and needle
- 2 alcohol swabs
- 1 sharps container for throwing away used needles and syringes. See "Disposing of used needles and syringes" at the end of these instructions.
Preparing your Lantus dose:
- Wash your hands with soap and water or with alcohol.
- Check the Lantus label to make sure you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- Check the insulin to make sure it is clear and colorless. Do not use Lantus if it is colored or cloudy, or if you see particles in the solution.
- Do not use Lantus after the expiration date stamped on the label or 28 days after you first use it.
- Always use a syringe that is marked for U-100 insulin. If you use a syringe other than a U-100 insulin syringe, you may get the wrong dose of insulin.
- Always use a new syringe or needle for each injection. Do not re-use or share your syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
Step 1:
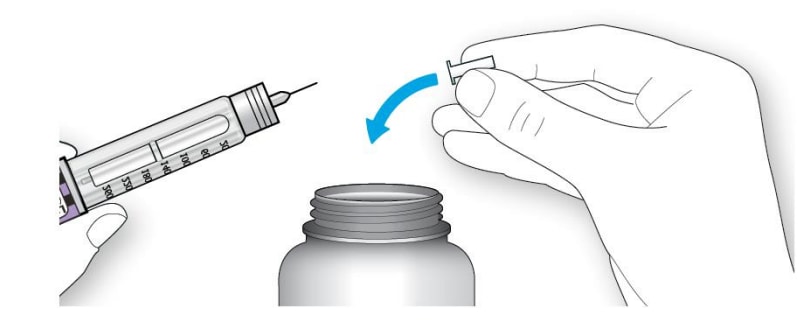
If you are using a new vial, remove the protective cap. Do not remove the stopper.
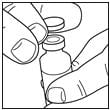
Step 2:
Wipe the top of the vial with an alcohol swab. You do not have to shake the vial of Lantus before use.
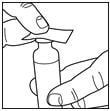
Step 3:
Draw air into the syringe equal to your insulin dose. Put the needle through the rubber top of the vial and push the plunger to inject the air into the vial.
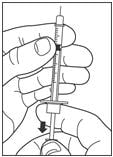
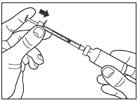
Step 4:
Leave the syringe in the vial and turn both upside down. Hold the syringe and vial firmly in one hand. Make sure the tip of the needle is in the insulin. With your free hand, pull the plunger to withdraw the correct dose into the syringe.
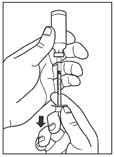
Step 5:
Before you take the needle out of the vial, check the syringe for air bubbles. If bubbles are in the syringe, hold the syringe straight up and tap the side of the syringe until the bubbles float to the top. Push the bubbles out with the plunger and draw insulin back in until you have the correct dose.
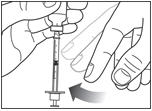
Step 6:
Remove the needle from the vial. Do not let the needle touch anything. You are now ready to inject.
Injecting Lantus:
- Inject your insulin exactly as your healthcare provider has shown you.
- Lantus is injected once daily at any time of the day but at the same time every day
Step 7:
Choosing your injection site:
- Lantus is injected under the skin (subcutaneously) of your upper arm, thigh, or stomach area (abdomen).
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in the skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.

- Wipe the skin with an alcohol swab to clean the injection site. Let the injection site dry before you inject your dose.
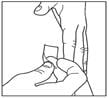
Step 8:
- Pinch the skin.
- Insert the needle in the way your healthcare provider showed you.
- Release the skin.
- Slowly push in the plunger of the syringe all the way, making sure you have injected all the insulin.
- Leave the needle in the skin for about 10 seconds.
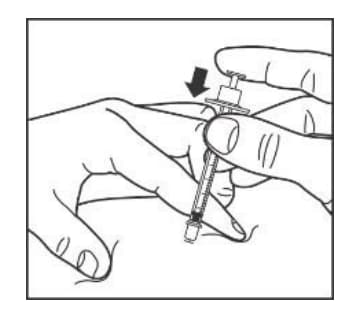
Step 9:
- Pull the needle straight out of your skin.
- Gently press the injection site for several seconds. Do not rub the area.
- Do not recap the used needle. Recapping the needle can lead to a needle stick injury.
Disposing of Used Needles and Syringes
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Storing and Disposing of Lantus?
Unopened (not in-use) Lantus vials
- Store unused Lantus vials in the refrigerator from 36°F to 46°F (2°C to 8°C).
- Do not freeze Lantus.
- Keep Lantus away from direct heat and light.
- If a vial has been frozen or overheated, throw it away.
- Unopened vials can be used until the expiration date on the carton and vial label if they have been stored in the refrigerator (they can be stored past 28 days in the refrigerator). •
- Unopened vials should be thrown away after 28 days if they are stored at room temperature.
After Lantus vials have been opened (in-use)
- Store in-use (opened) Lantus vials in a refrigerator from 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C) for up to 28 days.
- Do not freeze Lantus. If a vial has been frozen, throw it away.
- Keep Lantus out of direct heat and light.
- The Lantus vial you are using should be thrown away after 28 days or if the expiration date has passed, even if it still has insulin left in it.
Instructions for use revised 06/2022.