Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Solution, Injection [strength expressed as base, preservative free]:
Generic: 100 mg/10 mL (10 mL); 500 mg/50 mL (50 mL)
Solution Reconstituted, Injection [strength expressed as base]:
Generic: 100 mg (1 ea)
Solution Reconstituted, Injection [strength expressed as base, preservative free]:
Generic: 50 mg (1 ea); 100 mg (1 ea); 200 mg (1 ea); 350 mg (1 ea); 500 mg (1 ea)
Tablet, Oral [strength expressed as base]:
Generic: 5 mg, 10 mg, 15 mg, 25 mg
Pharmacology
Mechanism of Action
Leucovorin calcium is a reduced form of folic acid, leucovorin supplies the necessary cofactor blocked by methotrexate. Leucovorin actively competes with methotrexate for transport sites, displaces methotrexate from intracellular binding sites, and restores active folate stores required for DNA/RNA synthesis. Leucovorin stabilizes the binding of 5-dUMP and thymidylate synthetase, enhancing the activity of fluorouracil. When administered with pyrimethamine for the treatment of opportunistic infections, leucovorin reduces the risk for hematologic toxicity (HHS [OI adult 2019]).
Methanol toxicity treatment: Formic acid (methanol’s toxic metabolite) is normally metabolized to carbon dioxide and water by 10-formyltetrahydrofolate dehydrogenase after being bound to tetrahydrofolate. Administering a source of tetrahydrofolate may aid the body in eliminating formic acid (AACT [Barceloux] 2002).
Pharmacokinetics/Pharmacodynamics
Absorption
Oral, IM: Well absorbed
Metabolism
Intestinal mucosa and hepatically to 5-methyl-tetrahydrofolate (5MTHF; active)
Excretion
Urine (primarily); feces
Time to Peak
Oral: ~2 hours; IV: Total folates: 10 minutes; 5MTHF: ~1 hour; IM: Total folates: 52 minutes; 5MTHF: 2.8 hours
Half-Life Elimination
~4 to 8 hours
Use: Labeled Indications
Colorectal cancer, advanced: Injection: Palliative treatment of advanced colorectal cancer to prolong survival (in combination with fluorouracil).
Megaloblastic anemia: Injection: Treatment of megaloblastic anemias due to folic acid deficiency (when oral therapy is not feasible).
Methotrexate toxicity:
Injection: Rescue agent after high-dose methotrexate treatment in osteosarcoma and to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent overdosage of folic acid antagonists.
Oral: Rescue agent to diminish toxicity and counteract effects of impaired methotrexate elimination and inadvertent overdoses of folic acid antagonists.
Use: Off Label
Bladder cancer (neoadjuvant treatment)a
Data from a large, international, phase 3 study support the use of leucovorin calcium (as methotrexate rescue; in combination with cisplatin, vinblastine, and methotrexate) for neoadjuvant treatment of muscle invasive bladder cancer Griffiths 2011.
Dermatomyositis/polymyositisc
Methotrexate may be of benefit in the management of dermatomyositis/polymyositis; concomitant folic acid is recommended to reduce side effects associated with methotrexate. Leucovorin calcium rescue may be administered in patients who do not respond to folic acid Briemberg 2003, Miller 2019.
Esophageal cancer (advanced or metastatic)a
Data from a randomized, phase 3 study support the use of leucovorin calcium (in combination with fluorouracil and irinotecan [FOLFIRI]) for the treatment of locally advanced or metastatic adenocarcinoma of the esophagogastric junction Guimbaud 2014. Data from a randomized, phase 3 study support the use of leucovorin calcium (in combination with fluorouracil and oxaliplatin) for the treatment of locally advanced or metastatic adenocarcinoma of the esophagogastric junction Al-Batran 2008.
Gastric cancer (advanced or metastatic)a
Data from a randomized, phase 3 study support the use of leucovorin calcium (in combination with fluorouracil and irinotecan [FOLFIRI]) for the treatment of locally advanced or metastatic gastric adenocarcinoma Guimbaud 2014. Data from a randomized, phase 3 study support the use of leucovorin calcium (in combination with fluorouracil and oxaliplatin) for the treatment of locally advanced or metastatic gastric cancer Al-Batran 2008.
Gestational trophoblastic neoplasia (high risk)b
Data from several studies in patients with high-risk metastatic gestational trophoblastic neoplasia support the use of leucovorin calcium (in combination with etoposide, methotrexate, dactinomycin, cyclophosphamide, and vincristine [EMA-CO regimen] or with etoposide, methotrexate, dactinomycin, and cisplatin [EMA-EP regimen or EP-EMA regimen]) in the treatment of high-risk disease Escobar 2003, Ghaemmaghami 2004, Lurain 2006, Newlands 2000.
Graft-vs-host disease, acute (prophylaxis)yes
Based on the Prophylaxis and Treatment of GVHD: EBMT-ELN Working Group Recommendations for a Standardized Practice, methotrexate (with leucovorin calcium rescue) as combination therapy given to prevent acute graft-vs-host disease is effective and recommended in the prevention of this condition EBMT-ELN [Ruutu 2013].
Granulomatosis with polyangiitis (Wegener granulomatosis) and microscopic polyangiitis (maintenance therapy after remission induction)b
Data from an open-label, randomized, multicenter study comparing methotrexate to azathioprine support the use of oral methotrexate (with leucovorin calcium rescue) as maintenance therapy (once remission induction was achieved) in the management of granulomatosis with polyangiitis and microscopic polyangiitis Pagnoux 2008. Another open-label, prospective study also supports the use of oral methotrexate (with leucovorin calcium rescue) as maintenance therapy for managing granulomatosis with polyangiitis and microscopic polyangiitis following remission induction Langford 2003.
Hepatobiliary cancers (advanced)c
Data from a small, phase 2 study support the use of leucovorin calcium (in combination with gemcitabine and fluorouracil) in the management of unresectable or metastatic biliary tract and gall bladder adenocarcinoma Alberts 2005.
Methanol toxicity (adjunctive cofactor therapy)yes
Based on the American Academy of Clinical Toxicology guidelines for the treatment of methanol poisoning, leucovorin calcium given as adjunctive cofactor therapy to aid in the elimination of formic acid in methanol-poisoned patients is effective and recommended in the management of these patients AACT [Barceloux 2002].
Non-Hodgkin lymphomab
Data from several studies support the use of leucovorin calcium rescue (when administered with methotrexate-containing chemotherapy regimens) in the management of Burkitt non-Hodgkin lymphoma Khouri 1998, Mead 2008, Thomas 2006.
Nonleukemic meningeal cancerb
Data from a randomized, controlled study support the use of intrathecal methotrexate (with leucovorin calcium rescue) in the treatment of nonleukemic meningeal cancer Glantz 1999.
Pancreatic cancer (advanced or metastatic)ayes
Data from a phase 2/3, randomized trial support the use of leucovorin calcium (in combination with fluorouracil, oxaliplatin, and irinotecan; FOLFIRINOX regimen) in the management of patients with metastatic pancreatic cancer Conroy 2011.
According to the American Society of Clinical Oncology (ASCO) Guidelines for Metastatic Pancreatic Cancer, leucovorin, as part of the FOLFIRINOX regimen (fluorouracil, leucovorin, oxaliplatin, and irinotecan) is recommended as first-line therapy in patients with Eastern Cooperative Oncology Group performance status of 0 or 1, a favorable comorbidity profile, a preference for aggressive therapy, a suitable support system, and access to a chemotherapy port/infusion pump management service. For patients with a performance status of 2 or comorbidities, fluorouracil (with leucovorin) may be considered as an option for second-line therapy.
According to the ASCO guidelines for locally advanced, unresectable pancreatic cancer, induction with 6 months of initial systemic therapy (with a combination regimen) is generally recommended, although there is not enough evidence to encourage one regimen over another. If disease progression occurs, treatment according to guidelines for metastatic pancreatic cancer should be offered.
Pancreatic cancer, potentially curable, adjuvant therapyayes
Data from a multicenter, randomized, phase 3 study support the use of leucovorin calcium (in combination with fluorouracil, irinotecan, and oxaliplatin [modified FOLFIRINOX regimen]) as adjuvant therapy following complete resection of pancreatic ductal adenocarcinoma Conroy 2018. Data from 2 multicenter, randomized, phase 3 studies support the use of leucovorin calcium (with fluorouracil) in the treatment of resected pancreatic cancer Neoptolemos 2004, Neoptolemos 2010.
According to the ASCO Guidelines for Potentially Curable Pancreatic Cancer, leucovorin, as part of the modified FOLFIRINOX regimen (fluorouracil, leucovorin, oxaliplatin, and irinotecan) is the preferred adjuvant therapy in patients without concerns for toxicity or tolerance and in the absence of medical or surgical contraindications. Alternatively, if there are concerns of toxicity or tolerance, fluorouracil plus leucovorin calcium is an option that may be offered.
Prevention of pyrimethamine hematologic toxicity in HIV-infected patientsyes
Based on the US Department of Health and Human Services (HHS) Guidelines for Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV, leucovorin calcium is effective and recommended for the prevention of pyrimethamine hematologic toxicity in patients with HIV.
Primary CNS lymphoma (newly diagnosed)b
Data from a multicenter, phase 2 trial support the use of methotrexate with leucovorin calcium rescue (in combination with rituximab, procarbazine, and vincristine [R-MPV], followed by radiotherapy and cytarabine, and intrathecal/intra-Ommaya methotrexate) for the treatment of newly diagnosed primary CNS lymphoma (PCNSL) Shah 2007. Data from a small, phase 2 study support the use of methotrexate with leucovorin calcium rescue (in combination with temozolomide and rituximab and followed by high-dose consolidation chemotherapy) for the treatment of newly diagnosed PCNSL Rubenstein 2013. Data from the phase 2 portion of a phase 1-2 study also support the use of methotrexate with leucovorin calcium rescue (in combination with temozolomide and rituximab and followed by whole brain radiation therapy and subsequent temozolomide) for the treatment of newly diagnosed PCNSL Glass 2016.
Tubal ectopic pregnancycyes
Evidence from multiple case series Barnhart 2003, Ory 1986, Sauer 1987 and a prospective nonrandomized trial Stovall 1989 supports the use of leucovorin calcium (as a rescue in combination with a multidose methotrexate regimen) for the medical management of tubal ectopic pregnancy in appropriately selected patients.
Based on the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin: Tubal Ectopic Pregnancy ACOG 193 2018 and the American Society for Reproductive Medicine: Medical Treatment of Ectopic Pregnancy: A Committee Opinion ASRM 2013, leucovorin calcium (as a rescue in combination with a multidose methotrexate regimen) is effective and recommended in the management of tubal ectopic pregnancy. Methotrexate treatment may be appropriate in hemodynamically stable females with a nonruptured tubal ectopic pregnancy and who do not have absolute contraindications to methotrexate administration specific to this indication ACOG 193 2018. Refer to the Methotrexate monograph for additional information, including absolute and relative methotrexate contraindications.
Contraindications
Pernicious anemia and other megaloblastic anemias secondary to vitamin B12-deficiency
Canadian labeling: Additional contraindications (not in the US labeling): Hypersensitivity to leucovorin or any component of the formulation; intrathecal administration
Dosage and Administration
Dosing: Adult
Note: Because oral absorption is saturable at doses >25 mg, administering single oral doses >25 mg is not recommended (convert to parenteral therapy). Leucovorin calcium is a substrate for glucarpidase and may compete with methotrexate for binding sites; when administering glucarpidase concomitantly with leucovorin calcium, administer leucovorin calcium at least 2 hours before or 2 hours after the glucarpidase dose (Ramsey 2018). Do not administer leucovorin intrathecally; the use of intrathecal leucovorin is not advised (Jardine 1996; Smith 2008).
Bladder cancer, neoadjuvant treatment (off-label use): IV, Oral: 15 mg every 6 hours for 4 doses on days 2 and 9, starting 24 hours after each methotrexate dose (in combination with methotrexate, vinblastine, and cisplatin) (Griffiths 2011).
Colorectal cancer, advanced: IV: 200 mg/m2/day over at least 3 minutes for 5 days every 4 weeks for 2 cycles, then every 4 to 5 weeks (in combination with fluorouracil) or 20 mg/m2/day for 5 days every 4 weeks for 2 cycles, then every 4 to 5 weeks (in combination with fluorouracil). Note: Multiple leucovorin-containing regimens are available for the treatment of colorectal cancer. Refer to appropriate literature/guidelines for additional details.
Roswell Park regimen (off-label dosing): IV: 500 mg/m2 over 2 hours on days 1, 8, 15, 22, 29, and 36 (in combination with fluorouracil, administered 1 hour after the start of leucovorin) every 8 weeks for 4 cycles (Haller 2005).
FOLFOX6 and mFOLFOX6 regimen (off-label dosing): IV: 400 mg/m2 over 2 hours on day 1 or 350 mg (flat dose) over 2 hours on day 1 (in combination with fluorouracil and oxaliplatin) every 2 weeks until disease progression or unacceptable toxicity (Cheeseman 2002; Maindrault-Goebel 1999).
FOLFIRI regimen (off-label dosing): IV: 400 mg/m2 over 2 hours on day 1 every 2 weeks (in combination with fluorouracil and irinotecan) until disease progression or unacceptable toxicity (Andre 1999).
Dermatomyositis/polymyositis (off-label use): Oral: 5 mg once per week (administered 8 to 12 hours after the methotrexate dose) (Briemberg 2003; Miller 2019).
Esophageal cancer, advanced or metastatic (off-label use): IV: 400 mg/m2 over 2 hours once every 2 weeks (in combination with fluorouracil and irinotecan [FOLFIRI]) until disease progression or unacceptable toxicity (Guimbaud 2014) or 200 mg/m2 over 2 hours once every 2 weeks (in combination with fluorouracil and oxaliplatin) until disease progression or unacceptable toxicity (Al-Batran 2008).
Folic acid antagonist (eg, trimethoprim, pyrimethamine) overdose: Oral: 5 to 15 mg once daily.
Gastric cancer, advanced or metastatic (off-label use): IV: 400 mg/m2 over 2 hours once every 2 weeks (in combination with fluorouracil and irinotecan [FOLFIRI]) until disease progression or unacceptable toxicity (Guimbaud 2014) or 200 mg/m2 over 2 hours once every 2 weeks (in combination with fluorouracil and oxaliplatin) until disease progression or unacceptable toxicity (Al-Batran 2008).
Gestational trophoblastic neoplasia, high-risk metastatic disease (off-label use): IM, Oral: Note: The oral route would be preferred over IM in patients with thrombocytopenia.
EMA-CO regimen: 15 mg every 12 hours for 4 doses, beginning 24 hours after the start of methotrexate (in combination with etoposide, methotrexate, dactinomycin, cyclophosphamide, and vincristine); patients with brain metastases received a higher methotrexate dose and, therefore, the leucovorin calcium dose was increased to 30 mg every 12 hours for 6 doses, beginning 32 hours after the start of methotrexate. Continue for at least 2 treatment cycles after a normal human chorionic gonadotropin (hCG) level (Escobar 2003; Lurain 2006).
EMA-EP regimen: 15 mg every 12 hours for 4 doses beginning on day 2 (in combination with etoposide, methotrexate, dactinomycin, and cisplatin); patients with brain metastases received a higher methotrexate dose and, therefore, an increased leucovorin calcium dose of 30 mg every 12 hours. Continue for 2 to 4 treatment cycles after a normal hCG level (Ghaemmaghami 2004).
EP-EMA regimen: 15 mg every 12 hours for 4 doses, beginning 24 hours after the start of methotrexate (in combination with etoposide, cisplatin, methotrexate, and dactinomycin); if mucositis develops, may double the dose and duration of leucovorin calcium (Newlands 2000).
Graft-vs-host disease, acute (prophylaxis) (off-label use): IV, Oral: 15 mg every 6 hours for 3 doses on day 2, then 15 mg every 6 hours for 4 doses on days 4, 7, and 12 (in combination with methotrexate and cyclosporine); initiate leucovorin 24 hours after each methotrexate dose (methotrexate is administered on days 1, 3, 6, and 11; may omit day 11 methotrexate for ≥ grade 2 toxicity) (EBMT-ELN [Ruutu 2013]).
Granulomatosis with polyangiitis (Wegener granulomatosis) and microscopic polyangiitis (maintenance therapy after remission induction) (off-label use): Oral: 5 to 10 mg once weekly administered 24 hours after methotrexate (Langford 2003) or 25 mg once weekly administered 48 hours after methotrexate (Pagnoux 2008).
Hepatobiliary cancers, advanced (off-label use): IV: 25 mg/m2 immediately prior to fluorouracil infusion on days 1, 8, and 15 every 4 weeks (in combination with gemcitabine and fluorouracil) (Alberts 2005).
Megaloblastic anemia, folate-deficient: IM, IV: ≤1 mg once daily.
Methanol toxicity, adjunctive cofactor therapy (off-label use): IV: 1 mg/kg (maximum dose: 50 mg) over 30 to 60 minutes every 4 to 6 hours. Therapy should continue until methanol and formic acid have been completely eliminated (AACT [Barceloux 2002]).
Methotrexate-rescue (high-dose): Initial: Oral, IM, IV: 15 mg (~10 mg/m2); start 24 hours after beginning methotrexate infusion; continue every 6 hours for 10 doses, until methotrexate level is <0.05 micromolar. Monitor hydration and electrolyte status, as well as urine alkalinization. Adjust dose per institutional protocol or as follows:
Normal methotrexate elimination (serum methotrexate level ~10 micromolar at 24 hours after administration, 1 micromolar at 48 hours, and <0.2 micromolar at 72 hours): Oral, IM, IV: 15 mg every 6 hours for 60 hours (10 doses) beginning 24 hours after the start of methotrexate infusion
Delayed late methotrexate elimination (serum methotrexate level remaining >0.2 micromolar at 72 hours and >0.05 micromolar at 96 hours after administration): Continue leucovorin calcium 15 mg (oral, IM or IV) every 6 hours until methotrexate level is <0.05 micromolar
Delayed early methotrexate elimination and/or acute renal injury (serum methotrexate level ≥50 micromolar at 24 hours, or ≥5 micromolar at 48 hours, or a doubling of serum creatinine level at 24 hours after methotrexate administration): IV: 150 mg every 3 hours until methotrexate level is <1 micromolar, then 15 mg every 3 hours until methotrexate level is <0.05 micromolar
Methotrexate overexposure (high-dose): Leucovorin nomogram dosing for high-dose methotrexate overexposure (off-label dosing; generalized dosing derived from reference nomogram figures, refer to each reference [Bleyer 1978; Bleyer 1981; Widemann 2006] or institution-specific nomogram for details):
At 24 hours:
For methotrexate levels of ≥100 micromolar at ~24 hours, leucovorin calcium is initially dosed at 1,000 mg/m2 IV every 6 hours.
For methotrexate levels of ≥10 to <100 micromolar at 24 hours, leucovorin calcium is initially dosed at 100 mg/m2 IV every 3 or 6 hours.
For methotrexate levels of ~1 to 10 micromolar at 24 hours, leucovorin calcium is initially dosed at 10 mg/m2 IV or orally every 3 or 6 hours.
At 48 hours:
For methotrexate levels of ≥100 micromolar at 48 hours, leucovorin calcium is dosed at 1,000 mg/m2 IV every 6 hours.
For methotrexate levels of ≥10 to <100 micromolar at 48 hours, leucovorin calcium is dosed at 100 mg/m2 IV every 3 hours.
For methotrexate levels of ~1 to 10 micromolar at 48 hours, leucovorin calcium is dosed at 100 mg/m2 IV every 6 hours or 10 mg/m2 IV or orally to 100 mg/m2 IV every 3 hours.
At 72 hours:
For methotrexate levels of ≥10 micromolar at 72 hours, leucovorin calcium is dosed at 100 to 1,000 mg/m2 IV every 3 to 6 hours.
For methotrexate levels of ~1 to 10 micromolar at 72 hours, leucovorin calcium is dosed at 10 mg/m2 IV or orally to 100 mg/m2 IV every 3 hours.
For methotrexate levels of ~0.1 to 1 micromolar at 72 hours, leucovorin calcium is dosed at 10 mg/m2 IV or orally every 3 to 6 hours.
If serum creatinine is increased >50% above baseline, increase the standard leucovorin calcium dose to 100 mg/m2 IV every 3 hours, then adjust according to methotrexate levels above.
Follow methotrexate levels daily, leucovorin calcium may be discontinued when methotrexate level is <0.1 micromolar.
Some regimens use the following equation when calculating the leucovorin calcium dose (if the methotrexate plasma concentration is >5 micromolar) (Ramsey 2018):
Plasma methotrexate concentration (micromolar) × body weight (kg)
Methotrexate overdose (inadvertent) (begin as soon as possible after overdose): Oral, IM, IV: 10 mg/m2 every 6 hours until the methotrexate level is <0.01 micromolar. If serum creatinine is increased >50% above baseline 24 hours after methotrexate administration, if 24 hour methotrexate level is >5 micromolar, or if 48 hour methotrexate level is >0.9 micromolar, increase leucovorin dose to 100 mg/m2 IV every 3 hours until the methotrexate level is <0.01 micromolar.
Non-Hodgkin lymphoma, Burkitt (off-label use): IV, Oral:
CODOX-M/IVAC regimen: 15 mg/m2 IV every 3 hours for 5 doses beginning at hour 36 after the start of methotrexate, then 15 mg/m2 IV every 6 hours until methotrexate level is <0.05 micromolar. In addition, leucovorin calcium 15 mg (flat dose) orally is administered 24 hours after intrathecal methotrexate (Mead 2008). CODOX-M cycle (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate with leucovorin calcium rescue, intrathecal cytarabine, and intrathecal methotrexate) alternates with IVAC cycle (etoposide, ifosfamide, mesna, cytarabine, and intrathecal methotrexate (with leucovorin calcium rescue); refer to protocol for details.
Hyper-CVAD alternating with high-dose methotrexate/cytarabine regimen: 50 mg IV administered 12 hours after methotrexate completion, followed by 15 mg IV every 6 hours for 8 doses or until methotrexate level is <0.1 micromolar (adjust dose based on methotrexate level as needed) (Thomas 2006) or 50 mg IV administered 24 hours after the end of methotrexate infusion, followed by 15 mg orally every 6 hours for 8 doses (adjust dose based on methotrexate level as needed) (Khouri 1998). Therapy consists of alternating cycles of hyper-CVAD (cyclophosphamide, mesna, vincristine, doxorubicin, and dexamethasone) and high-dose methotrexate/cytarabine; refer to protocol for details.
Nonleukemic meningeal cancer (off-label use): Oral: 10 mg every 6 hours for 8 doses, beginning 24 hours after each intrathecal methotrexate dose (Glantz 1999).
Pancreatic cancer, advanced or metastatic (off-label use): IV: 400 mg/m2 over 2 hours once every 2 weeks (in combination with fluorouracil, oxaliplatin, and irinotecan [FOLFIRINOX]) for at least 6 months (Conroy 2011).
Pancreatic cancer, potentially curable, adjuvant therapy (off-label use): Note: American Society of Clinical Oncology (ASCO) guidelines recommend 6 months of adjuvant therapy if recovery is complete; if preoperative chemotherapy therapy was received, a total of 6 months of adjuvant therapy (including the preoperative regimen) is recommended (ASCO [Khorana 2019]).
mFOLFIRINOX regimen: IV: 400 mg/m2 over 2 hours once every 2 weeks (in combination with fluorouracil, irinotecan, and oxaliplatin; modified FOLFIRINOX regimen) for 24 weeks (Conroy 2018). According to ASCO guidelines, mFOLFIRINOX is the preferred first-line adjuvant regimen for potentially curable disease (ASCO [Khorana 2019]).
Leucovorin/fluorouracil: 20 mg/m2/day (bolus) days 1 to 5 every 28 days (in combination with fluorouracil) for 6 cycles (Neoptolemos 2004; Neoptolemos 2010). According to ASCO guidelines for potentially curable pancreatic cancer, fluorouracil/leucovorin may be considered as an alternative therapy if toxicity/tolerance is a concern (ASCO [Khorana 2019]).
Prevention of pyrimethamine hematologic toxicity in HIV-infected patients (off-label use; HHS [OI adult 2019]): Oral:
Cystoisosporiasis (formerly Isosporiasis) (Cystoisospora belli):
Treatment: 10 to 25 mg once daily (in combination with pyrimethamine).
Chronic maintenance (secondary prophylaxis): 5 to 10 mg once daily (in combination with pyrimethamine).
Pneumocystis pneumonia: Prophylaxis (primary and secondary): 25 mg once weekly (in combination with pyrimethamine and dapsone) or 10 mg once daily (in combination with pyrimethamine and atovaquone).
Toxoplasma gondii encephalitis:
Primary prophylaxis: 25 mg once weekly (in combination with pyrimethamine and dapsone) or 10 mg once daily (in combination with pyrimethamine and atovaquone).
Treatment: 10 to 25 mg once daily as part of an appropriate combination regimen. Note: May increase leucovorin to 50 mg once or twice daily in cases of pyrimethamine toxicity (rash, nausea, bone marrow suppression).
Chronic maintenance (secondary prophylaxis): 10 to 25 mg once daily (in combination with pyrimethamine with either sulfadiazine or clindamycin) or 10 mg once daily (in combination with pyrimethamine and atovaquone).
Primary CNS lymphoma (off-label use): IV, Oral: 20 to 25 mg every 6 hours beginning 24 hours after methotrexate infusion (in combination with rituximab, methotrexate, vincristine, and procarbazine, followed by radiotherapy, cytarabine, and intra-Ommaya methotrexate), continue for at least 72 hours or until methotrexate level is <0.1 micromolar; may increase dose to 40 mg every 4 hours for elevated methotrexate levels (Shah 2007) or 100 mg/m2 IV every 6 hours starting 24 hours after methotrexate infusion, continue until methotrexate level is <0.05 micromolar (in combination with methotrexate, temozolomide, and rituximab, followed by high-dose consolidation therapy) (Batchelor 2003; Rubenstein 2013) or 25 mg IV every 6 hours starting 24 hours after methotrexate, continue until appropriate methotrexate level is reached (in combination with methotrexate, temozolomide, and rituximab, followed by whole brain radiotherapy) (Glass 2016).
Tubal ectopic pregnancy (off-label use) (in combination with a multidose methotrexate regimen): IM: Methotrexate on days 1, 3, 5, and 7 alternating with leucovorin calcium 0.1 mg/kg on days 2, 4, 6, and 8. Measure serum hCG on each day of methotrexate administration. If serum hCG decreases by >15% from previous measurement, discontinue methotrexate and leucovorin (total treatment may be between 1 and 4 doses each). If serum hCG decreases by <15% from previous measurement, administer methotrexate (maximum 4 doses) and then leucovorin calcium the next day (ACOG 193 2018; ASRM 2013; Sauer 1987; Stovall 1989).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Note: Dosing in pediatric patients presented in mg, mg/m2 and mg/kg; use caution. Because oral absorption is saturable at doses >25 mg, administration of single oral doses >25 mg is not recommended (convert to parenteral therapy); also consider parenteral administration instead of oral in patients with GI toxicity, nausea, or vomiting.
Folic acid antagonist (eg, pyrimethamine, trimethoprim) overdose: Infant, Children, and Adolescents: Oral: 5 to 15 mg daily
Folinic-acid dependent seizures: Very limited data available: Infants and Children: Oral: Initial: 2.5 to 5 mg twice daily; may gradually increase for seizure recurrence; doses up to 8 mg/kg/day (ie, 25 mg three times daily) have been reported. Dosing based on small case series and case reports in patients with refractory seizures who were on multiple anticonvulsants with or without pyridoxine (Cloherty 2012; Djukic 2007; Frye 2003; Gallagher 2009; Hyland 1995; Nicolai 2006; Torres 1999)
Megaloblastic anemia secondary to folate deficiency: Infants, Children, and Adolescents: IM, IV: ≤1 mg/day
High-dose methotrexate rescue: Infants, Children, and Adolescents: Initial: Oral, IM, IV: 15 mg (~10 mg/m2) every 6 hours; start 24 hours after beginning of methotrexate infusion (based on a methotrexate dose of 12 to 15 g/m2 IV over 4 hours). Leucovorin (and hydration and urinary alkalinization) should be continued and/or adjusted until the methotrexate level is <0.05 micromolar (5 x 10-8 M). Adjust dose as follows:
Normal methotrexate elimination (serum methotrexate level ~10 micromolar at 24 hours after administration, 1 micromolar at 48 hours, and <0.2 micromolar at 72 hours): Oral, IM, IV: 15 mg every 6 hours for 10 doses beginning 24 hours after the start of methotrexate infusion
Delayed late methotrexate elimination (serum methotrexate level remaining >0.2 micromolar at 72 hours and >0.05 micromolar at 96 hours after administration): Oral, IM, IV: Continue leucovorin calcium 15 mg every 6 hours until methotrexate level is <0.05 micromolar
Delayed early methotrexate elimination and/or acute renal injury (serum methotrexate level ≥50 micromolar at 24 hours, or ≥5 micromolar at 48 hours, or a doubling of serum creatinine level at 24 hours after methotrexate administration): IV: 150 mg every 3 hours until methotrexate level is <1 micromolar, then 15 mg every 3 hours until methotrexate level is <0.05 micromolar
High-dose methotrexate overexposure: Limited data available: Infants, Children, and Adolescents: Leucovorin nomogram dosing for high-dose methotrexate overexposure (generalized dosing derived from reference nomogram figures, refer to each reference (Bleyer, 1978; Bleyer, 1981; Widemann, 2006) or protocol-specific nomogram for details):
At 24 hours:
For methotrexate levels of ≥100 micromolar at ~24 hours, leucovorin is initially dosed at 1,000 mg/m2 IV every 6 hours
For methotrexate levels of ≥10 to <100 micromolar at 24 hours, leucovorin is initially dosed at 100 mg/m2 IV every 3 or 6 hours
For methotrexate levels of ~1 to 10 micromolar at 24 hours, leucovorin is initially dosed at 10 mg/m2 IV or orally every 3 or 6 hours
At 48 hours:
For methotrexate levels of ≥100 micromolar at 48 hours, leucovorin is dosed at 1,000 mg/m2 IV every 6 hours
For methotrexate levels of ≥10 to <100 micromolar at 48 hours, leucovorin is dosed at 100 mg/m2 IV every 3 hours
For methotrexate levels of ~1 to 10 micromolar at 48 hours, leucovorin is dosed at 100 mg/m2 IV every 6 hours or 10 mg/m2 IV or orally to 100 mg/m2 IV every 3 hours
At 72 hours:
For methotrexate levels of ≥10 micromolar at 72 hours, leucovorin is dosed at 100 to 1,000 mg/m2 IV every 3 to 6 hours
For methotrexate levels of ~1 to 10 micromolar at 72 hours, leucovorin is dosed at 10 mg/m2 IV or orally to 100 mg/m2 IV every 3 hours
For methotrexate levels of ~0.1 to 1 micromolar at 72 hours, leucovorin is dosed at 10 mg/m2 IV or orally every 3 to 6 hours
If serum creatinine is increased more than 50% above baseline, increase the standard leucovorin dose to 100 mg/m2 IV every 3 hours, then adjust according to methotrexate levels above.
Follow methotrexate levels daily, leucovorin may be discontinued when methotrexate level is <0.1 micromolar; some have suggested <0.08 micromolar (8 x 10-8 M) is preferable (Bleyer, 1978)
Methotrexate overdose (inadvertent) (begin as soon as possible after overdose): Infants, Children, and Adolescents: Oral, IM, IV: 10 mg/m2/dose every 6 hours until the methotrexate level is <0.01 micromolar. If serum creatinine is increased more than 50% above baseline 24 hours after methotrexate administration, if 24 hour methotrexate level is >5 micromolar, or if 48 hour methotrexate level is >0.9 micromolar, increase leucovorin dose to 100 mg/m2 IV every 3 hours until the methotrexate level is <0.01 micromolar.
Note: Do not administer leucovorin intrathecally; use of intrathecal leucovorin is not advised (Jardine, 1996; Smith, 2008).
Megaloblastic anemia secondary to congenital deficiency of dihydrofolate reductase; treatment: Infants, Children, and Adolescents: IM, Oral: 3 to 6 mg/day (Nelson, 1996)
Pyrimethamine hematologic toxicity, prevention: Leucovorin is administered in combination with pyrmethamine and other medications in the treatment of toxoplasmosis; dosing varies with disease process and regimen; consult pyrimethamine specific monograph for additional details; duration of therapy varies based on disease state; however, leucovorin should continue for 1 week after pyrimethamine is discontinued (DHHS [pediatric], 2013); usual regimens include:
Toxoplasmosis (Toxoplasma gondii):
Treatment:
Congenital toxoplasmosis: Infants:
HIV-exposed/-positive: IM, Oral: 10 mg with every pyrimethamine dose; treatment duration: 12 months (DHHS [pediatric], 2013)
Non-HIV-exposed/-positive: IM, Oral: 10 mg three times weekly for 12 months (McAuley, 2008)
Acquired infection:
HIV-exposed/-positive: Infants, Children, and Adolescents: Acute induction: Oral: 10 to 25 mg once daily for ≥6 weeks (DHHS, 2014; DHHS [pediatric], 2013). Note: In adolescents, leucovorin may be increased to 50 to100 mg/day in divided doses in cases of pyrimethamine toxicity (rash, nausea, bone marrow suppression) (DHHS, 2014)
Non-HIV-exposed/-positive: Chorioretinitis: Infants, Children, and Adolescents: Oral: 10 to 20 mg three times weekly (McAuley, 2008)
Prophylaxis:
Primary:
HIV-exposed/-positive:
Infants and Children: Oral: 5 mg once every 3 days (DHHS [pediatric], 2013)
Adolescents: Oral: 25 mg once weekly (with pyrimethamine and dapsone) or 10 mg once daily (with pyrimethamine and atovaquone) (DHHS, 2014)
Hematopoietic stem cell transplantation recipients:
Children: Oral: 5 mg every 3 days; initiate after engraftment and administer as long as the patient remains on immunosuppressive therapy (Tomblyn, 2009)
Adolescents: Oral: 10 to 25 mg once daily; initiate after engraftment and administer as long as the patient remains on immunosuppressive therapy (Tomblyn, 2009)
Secondary: HIV-exposed/-positive:
Infants and Children: Oral: 5 mg once every 3 days (DHHS [pediatric], 2013)
Adolescents: Oral: 10 to 25 mg once daily or 10 mg once daily (DHHS, 2014)
Pneumocystis jirovecii pneumonia (PCP): Prophylaxis (primary and secondary): Adolescents: Oral: 25 mg once weekly or 10 mg once daily (DHHS, 2014)
Isosporiasis (Isospora belli):
Treatment, acute infection: Infants, Children, and Adolescents: Oral: 10 to 25 mg once daily (DHHS, 2014; DHHS [pediatric], 2013)
Prophylaxis, secondary:
Infants and Children: Oral: 10 to 25 mg once daily (DHHS [pediatric], 2013)
Adolescents: Oral: 5 to10 mg once daily (DHHS, 2014)
Methanol toxicity; cofactor therapy: Limited data available: Infants, Children, and Adolescents: IV: 1 mg/kg (maximum dose: 50 mg) over 30 to 60 minutes every 4 to 6 hours. Therapy should continue until methanol and formic acid have been completely eliminated (Barceloux, 2002).
Reconstitution
Powder for injection: Reconstitute with SWFI or BWFI (refer to specific product labeling for details); dilute in D5W or NS for infusion. When doses >10 mg/m2 are required, reconstitute using SWFI, not a solution containing benzyl alcohol.
For methanol toxicity, dilute in D5W (AACT [Barceloux] 2002).
Extemporaneously Prepared
A 5 mg/mL oral suspension may be prepared with tablets, Cologel, and a 2:1 mixture of simple syrup and wild cherry syrup. Crush twenty-four 25 mg tablets in a glass mortar and reduce to a fine powder; transfer powder to amber bottle. Add 30 mL Cologel and shake mixture thoroughly. Add a quantity of syrup mixture sufficient to make 120 mL. Label “shake well” and “refrigerate”. Stable for 28 days refrigerated.
Lam MS. Extemporaneous Compounding of Oral Liquid Dosage Formulations and Alternative Drug Delivery Methods for Anticancer Drugs. Pharmacotherapy. 2011;31(2):164-192.21275495
Administration
Due to calcium content, do not administer IV solutions at a rate >160 mg/minute; not intended for intrathecal use.
Refer to individual protocols. Should be administered IM, IV push, or IV infusion (15 minutes to 2 hours). Leucovorin should not be administered concurrently with methotrexate. It is commonly initiated 24 hours after the start of methotrexate. Leucovorin calcium is a substrate for glucarpidase and may compete with methotrexate for binding sites; when administering glucarpidase concomitantly with leucovorin calcium, administer leucovorin calcium at least 2 hours before or 2 hours after the glucarpidase dose (Ramsey 2018).
As a rescue after folate antagonists: Administer by IV bolus, IM, or orally.
Do not administer orally in the presence of nausea or vomiting. Because oral absorption is saturable at doses >25 mg, administering oral doses >25 mg is not recommended (convert to parenteral therapy).
Combination therapy with fluorouracil: Fluorouracil is usually given after, or at the midpoint, of the leucovorin infusion. Leucovorin is usually administered by IV bolus injection or short (10 to 120 minutes) IV infusion. Other administration schedules have been used; refer to individual protocols.
For the treatment of methanol toxicity, infuse over 30 to 60 minutes (AACT [Barceloux 2002]).
Dietary Considerations
Solutions for injection contain calcium 0.004 mEq per leucovorin 1 mg
Storage
Powder for injection: Powder for injection: Store intact vials and reconstituted solution at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). Protect from light. Solutions reconstituted with bacteriostatic water for injection USP must be used within 7 days. Solutions reconstituted with SWFI must be used immediately.
Solution for injection: Prior to dilution, store vials at 2°C to 8°C (36°F to 46°F). Protect from light.
Tablet: Store at 20°C to 25°C (68°F to 77°F). Protect from light and moisture.
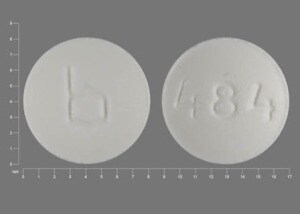
Leucovorin Calcium Images
Drug Interactions
Fluorouracil (Topical): Leucovorin Calcium-Levoleucovorin may enhance the adverse/toxic effect of Fluorouracil (Topical). Monitor therapy
Fluorouracil Products: Leucovorin Calcium-Levoleucovorin may enhance the adverse/toxic effect of Fluorouracil Products. Monitor therapy
Fosphenytoin: Leucovorin Calcium-Levoleucovorin may decrease the serum concentration of Fosphenytoin. Monitor therapy
Glucarpidase: May decrease serum concentrations of the active metabolite(s) of Leucovorin Calcium-Levoleucovorin. Specifically, 6S-5-methyltetrahydrofolateconcentrations may be reduced. Glucarpidase may decrease the serum concentration of Leucovorin Calcium-Levoleucovorin. Management: Avoid leucovorin administration within 2 hours of glucarpidase dosing. Continue to administer the pre-glucarpidase leucovorin dose for at least the first 48 hours after glucarpidase administration, and dose based on methotrexate concentration thereafter. Consider therapy modification
PHENobarbital: Leucovorin Calcium-Levoleucovorin may decrease the serum concentration of PHENobarbital. Monitor therapy
Phenytoin: Leucovorin Calcium-Levoleucovorin may decrease the serum concentration of Phenytoin. Monitor therapy
Primidone: Leucovorin Calcium-Levoleucovorin may decrease the serum concentration of Primidone. Additionally, leucovorin/levoleucovorin may decrease concentrations of active metabolites of primidone (e.g., phenobarbital). Monitor therapy
Raltitrexed: Leucovorin Calcium-Levoleucovorin may diminish the therapeutic effect of Raltitrexed. Avoid combination
Trimethoprim: Leucovorin Calcium-Levoleucovorin may diminish the therapeutic effect of Trimethoprim. Management: Avoid concurrent use of leucovorin or levoleucovorin with trimethoprim (plus sulfamethoxazole) for Pneumocystis jirovecii pneumonia. If trimethoprim is used for another indication, monitor closely for reduced efficacy. Avoid combination
Adverse Reactions
As reported in combination with 5-fluorouracil.
>10%:
Central nervous system: Fatigue (≤13%), lethargy (≤13%), malaise (≤13%)
Dermatologic: Alopecia (42% to 43%), dermatitis (21% to 25%)
Gastrointestinal: Stomatitis (75% to 84%; grades ≥3: 27% to 29%), nausea (74% to 80%), diarrhea (66% to 67%), vomiting (44% to 46%), anorexia (14% to 22%)
Miscellaneous: Drug toxicity
1% to 10%:
Gastrointestinal: Constipation (3% to 4%)
Infection: Infection (3% to 8%)
Frequency not defined: Gastrointestinal: Gastrointestinal toxicity
Postmarketing: Anaphylactic shock, anaphylaxis, hypersensitivity reaction, nonimmune anaphylaxis, seizure, syncope, urticaria
Warnings/Precautions
Concerns related to adverse effects:
- Hypersensitivity: Hypersensitivity, including allergic reactions, anaphylactoid reactions, and urticaria have been reported with leucovorin. Because leucovorin is typically administered in combination with other chemotherapy agents, it may be difficult to determine the causative agent for hypersensitivity reactions. In a series of 44 patients with hypersensitivity to leucovorin-containing regimens, hypersensitivity/infusion reaction to leucovorin was confirmed in 5 patients; reactions also occurred with subsequent rechallenge with LEVOleucovorin (Ureña-Tavera 2015).
- Seizures: Seizures or syncope have been reported (rarely) in patients with cancer receiving leucovorin, usually in association with fluoropyrimidine administration, and most commonly in patients with CNS metastases or other predisposing factors; a causal relationship has not been established.
Disease-related concerns:
- Anemias: Leucovorin is inappropriate treatment for pernicious anemia and other megaloblastic anemias secondary to a lack of vitamin B12; a hematologic remission may occur while neurologic manifestations progress.
- Renal impairment: Leucovorin is excreted renally; the risk for toxicities may be increased in patients with renal impairment.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
- Fluorouracil: Leucovorin may increase the toxicity of 5-fluorouracil; deaths from severe enterocolitis, diarrhea, and dehydration have been reported (in elderly patients); granulocytopenia and fever have also been reported.
- Sulfamethoxazole-trimethoprim: The combination of leucovorin and sulfamethoxazole-trimethoprim for the acute treatment of Pneumocystis jirovecii pneumonia in patients with HIV infection has been reported to cause increased rates of treatment failure.
Dosage form specific issues:
- Administration route: Parenteral administration may be preferred to oral if vomiting or malabsorption is likely. Because oral absorption is saturable at doses >25 mg, administering oral doses >25 mg is not recommended (convert to parenteral therapy).
- Benzyl alcohol and derivatives: When doses >10 mg/m2 are required using the powder for injection, reconstitute using sterile water for injection, not a solution containing benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC 1982); some data suggest that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol with caution in neonates. See manufacturer’s labeling.
- Injection: Due to calcium content, do not administer IV solutions at a rate >160 mg/minute. Not intended for intrathecal use.
Other warnings and precautions:
- Folic acid antagonist overdose: When used for the treatment of accidental folic acid antagonist overdose, administer as soon as possible.
- Methotrexate overdose: When used for the treatment of a methotrexate overdose, administer IV leucovorin as soon as possible. Monitoring of the serum methotrexate concentration is essential to determine the optimal dose/duration of leucovorin; however, do not wait for the results of a methotrexate level before initiating leucovorin. It is important to adjust the leucovorin dose once a methotrexate level is known. The dose may need to be increased or administration prolonged in situations in which methotrexate excretion may be delayed (eg, ascites, pleural effusion, renal insufficiency, inadequate hydration). Never administer leucovorin intrathecally.
- Methotrexate rescue therapy: Methotrexate serum concentrations should be monitored to determine dose and duration of leucovorin therapy. Dose may need to be increased or administration prolonged in situations where methotrexate excretion may be delayed (eg, ascites, pleural effusion, renal insufficiency, inadequate hydration). Never administer leucovorin intrathecally.
Monitoring Parameters
High-dose methotrexate therapy: Plasma methotrexate concentration; leucovorin is continued until the plasma methotrexate level <0.05 micromolar. With 4- to 6-hour high-dose methotrexate infusions, plasma drug values in excess of 50 and 1 micromolar at 24 and 48 hours after starting the infusion, respectively, are often predictive of delayed methotrexate clearance.
Fluorouracil therapy: CBC with differential and platelets, liver function tests, electrolytes
Tubal ectopic pregnancy (in combination with mulitdose methotrexate) (off-label use): Prior to therapy, serial hCG measurements 2 to 7 days apart along with other procedures to exclude the presence of a viable intrauterine pregnancy (ACOG 193 2018; RCOG [Elson 2016]). CBC with differential, liver function tests, serum creatinine, blood type and Rh (ASRM 2013). Evaluate for medical conditions considered absolute contraindications for methotrexate treatment of ectopic pregnancy (ACOG 193 2018). Measure serum hCG on days of methotrexate administration. Once decrease is >15%, continue to measure serum hCG weekly until reaching a non-pregnant level. Consider surgical management if serum hCG does not decrease after 4 doses (ACOG 193 2018).
Pregnancy
Pregnancy Risk Factor
C
Pregnancy Considerations
Animal reproduction studies have not been conducted. Leucovorin is a biologically active form of folic acid. Adequate amounts of folic acid are recommended during pregnancy. Refer to Folic Acid monograph.
Patient Education
What is this drug used for?
- It is used with methotrexate to avoid side effects.
- It may lower the side effects of some drugs.
- It is used with fluorouracil to help fight colorectal cancer.
- It is used to help with some kinds of anemia.
- It may be given to you for other reasons. Talk with the doctor.
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Seizures
- Severe dizziness
- Passing out
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.