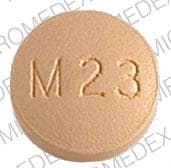
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Solution, Intravenous, as hydrochloride:
Generic: 250 mg/5 mL (5 mL [DSC])
Tablet, Oral:
Generic: 250 mg, 500 mg
Pharmacology
Mechanism of Action
Stimulation of central alpha-adrenergic receptors by a false neurotransmitter (alpha-methylnorepinephrine) that results in a decreased sympathetic outflow to the heart, kidneys, and peripheral vasculature
Pharmacokinetics/Pharmacodynamics
Absorption
Oral: Incomplete due to presystemic gut metabolism (Skerjanec 1995)
Distribution
Vd: 0.23 L/kg (Myhre 1982)
Metabolism
Intestinal and hepatic
Excretion
Urine (~70% as parent drug and metabolites); excretion complete within 36 hours
Onset of Action
Peak effect: Hypotensive: Oral, IV: Single-dose: Within 3 to 6 hours; Multiple-dose: 48 to 72 hours
Time to Peak
Plasma: Oral: 2 to 4 hours (Myhre 1982)
Duration of Action
Oral: Single-dose: 12 to 24 hours, Multiple-dose: 24 to 48 hours; IV: 10 to 16 hours
Half-Life Elimination
Neonates: 10 to 20 hours; Adults:1.5 to 2 hours; End-stage renal disease: Prolonged (Myhre 1982)
Protein Binding
10% to 15% (Myhre 1982)
Use: Labeled Indications
Hypertension: Management of hypertension. Note: Not recommended for the initial treatment of hypertension (ACC/AHA [Whelton 2017]).
Contraindications
Hypersensitivity to methyldopa or any component of the formulation; active hepatic disease (eg, acute hepatitis, active cirrhosis); hepatic disorders previously associated with use of methyldopa; concurrent use of MAO inhibitors
Dosage and Administration
Dosing: Adult
Note: Methyldopate injection is no longer available in the US.
Hypertension (alternative agent):
Oral: Initial: 250 mg 2 to 3 times daily; increase or decrease daily dose every 2 days based on response; maximum dose: 3,000 mg/day; usual dose range: 250 to 1,000 mg daily in 2 divided doses (ACC/AHA [Whelton 2017]). Note: When administered with other antihypertensives other than thiazide diuretics, limit initial daily dose of methyldopa to 500 mg/day.
IV: 250 to 1,000 mg every 6 to 8 hours; maximum: 1,000 mg every 6 hours
Dosing: Geriatric
Avoid use (Beers Criteria [AGS 2019]).
Dosing: Pediatric
Note: Methyldopate injection is no longer available in the US.
Hypertension: Note: Use has been replaced by other agents; methyldopa not suggested as a treatment option for hypertension (NHLBI 2011). Children and Adolescents: Oral: Initial: 10 mg/kg/day in 2 to 4 divided doses; titrate no more frequently than every 2 days until adequate response to maximum daily dose: 65 mg/kg/day or 3,000 mg/day whichever is less (Park 2014)
Hypertensive crisis: Note: Use has been replaced by other agents; methyldopa not suggested by experts as a treatment option (Flynn 2009; NHBPEP 2004; Thomas 2011): Children and Adolescents: IV: Initial: 2 to 4 mg/kg/dose every 6 to 8 hours; titrate as needed to a recommended daily dose of 20 to 40 mg/kg/day in divided doses or higher if needed up to maximum daily dose: 65 mg/kg/day or 3,000 mg/day whichever is less (Park 2014)
Reconstitution
Standard diluent: 250 to 500 mg/100 mL D5W
Extemporaneously Prepared
A 50 mg/mL oral suspension may be made with tablets and either unpreserved Simple Syrup, N.F. or a 1:1 mixture of simple syrup (containing 0.5% citric acid) and hydrochloric acid 0.2 N. Crush ten 250 mg tablets in a glass mortar and reduce to a fine powder. To make formulation with unpreserved simple syrup, add small portions of vehicle and mix to a uniform paste; mix while adding the vehicle in incremental proportions to almost 50 mL; transfer to a calibrated bottle; rinse the mortar and pestle several times with vehicle, and add quantity of vehicle sufficient to make 50 mL. To make formulation with the second vehicle, mix powdered tablets with 25 mL of hydrochloric acid 0.2 N (0.73% w/v); dilute this mixture to 50 mL with simple syrup containing 0.5% citric acid by the method described above. Label "shake well" and "protect from light." Stable for 14 days when stored in glass prescription bottles in the dark at room temperature or refrigerated.
Newton DW, Rogers AG, Becker CH, et al, "Extemporaneous Preparation of Methyldopa in Two Syrup Vehicles," Am J Hosp Pharm, 1975, 32(8):817-21.238389
Administration
IV: Infuse over 30 to 60 minutes.
Oral: Administer new dosage increases in the evening to minimize sedation.
Dietary Considerations
Dietary requirements for vitamin B12 and folate may be increased with high doses of methyldopa.
Storage
Injection: Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Injectable dosage form is most stable at acid to neutral pH. Stability of parenteral admixture in D5W at room temperature (25°C) is 24 hours. Parenteral admixture is stable at room temperature for up to 125 hours (Newton 1981).
Tablets: Store at 20°C to 25°C (68°F to 77°F). Protect from light.
Methyldopa Images
Drug Interactions
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Beta-Blockers: Alpha2-Agonists may enhance the AV-blocking effect of Beta-Blockers. Sinus node dysfunction may also be enhanced. Beta-Blockers may enhance the rebound hypertensive effect of Alpha2-Agonists. This effect can occur when the Alpha2-Agonist is abruptly withdrawn. Management: Closely monitor heart rate during treatment with a beta blocker and clonidine. Withdraw beta blockers several days before clonidine withdrawal when possible, and monitor blood pressure closely. Recommendations for other alpha2-agonists are unavailable. Exceptions: Levobunolol; Metipranolol. Consider therapy modification
Bradycardia-Causing Agents: May enhance the bradycardic effect of other Bradycardia-Causing Agents. Monitor therapy
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Ceritinib: Bradycardia-Causing Agents may enhance the bradycardic effect of Ceritinib. Management: If this combination cannot be avoided, monitor patients for evidence of symptomatic bradycardia, and closely monitor blood pressure and heart rate during therapy. Exceptions are discussed in separate monographs. Consider therapy modification
COMT Inhibitors: May decrease the metabolism of COMT Substrates. Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
Fexinidazole [INT]: Bradycardia-Causing Agents may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Iobenguane Radiopharmaceutical Products: Methyldopa may diminish the therapeutic effect of Iobenguane Radiopharmaceutical Products. Management: Discontinue all drugs that may inhibit or interfere with catecholamine transport or uptake for at least 5 biological half-lives before iobenguane administration. Do not administer methyldopa until at least 7 days after each iobenguane dose. Avoid combination
Iron Preparations: May decrease the serum concentration of Methyldopa. Exceptions: Ferric Carboxymaltose; Ferric Derisomaltose; Ferric Gluconate; Ferric Hydroxide Polymaltose Complex; Ferric Pyrophosphate Citrate; Ferumoxytol; Iron Dextran Complex; Iron Sucrose. Consider therapy modification
Ivabradine: Bradycardia-Causing Agents may enhance the bradycardic effect of Ivabradine. Monitor therapy
Lacosamide: Bradycardia-Causing Agents may enhance the AV-blocking effect of Lacosamide. Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Lithium: Methyldopa may enhance the adverse/toxic effect of Lithium. This may occur without notable changes in serum lithium concentrations. Monitor therapy
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Midodrine: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Mirtazapine: May diminish the antihypertensive effect of Alpha2-Agonists. Management: Consider avoiding concurrent use. If the combination cannot be avoided, monitor for decreased effects of alpha2-agonists if mirtazapine is initiated/dose increased, or increased effects if mirtazapine is discontinued/dose decreased. Consider therapy modification
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Monoamine Oxidase Inhibitors: May enhance the adverse/toxic effect of Methyldopa. Avoid combination
Multivitamins/Minerals (with ADEK, Folate, Iron): May decrease the serum concentration of Methyldopa. Management: Consider separating doses of these products by 2 or more hours to minimize this interaction; however, the success of this action appears limited. Monitor for decreased therapeutic effects of methyldopa with concurrent use. Consider therapy modification
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Riluzole: Methyldopa may enhance the adverse/toxic effect of Riluzole. Specifically, the risk of hepatotoxicity may be increased. Management: Consider alternatives to methyldopa in patients receiving treatment with riluzole due to the potential for additive hepatotoxicity. Consider therapy modification
Ruxolitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Management: Ruxolitinib Canadian product labeling recommends avoiding use with bradycardia-causing agents to the extent possible. Monitor therapy
Serotonin/Norepinephrine Reuptake Inhibitors: May diminish the antihypertensive effect of Alpha2-Agonists. Monitor therapy
Siponimod: Bradycardia-Causing Agents may enhance the bradycardic effect of Siponimod. Management: Avoid coadministration of siponimod with drugs that may cause bradycardia. Consider therapy modification
Terlipressin: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Tofacitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Tricyclic Antidepressants: May diminish the antihypertensive effect of Alpha2-Agonists. Management: Consider avoiding this combination. If used, monitor for decreased effects of the alpha2-agonist. Exercise great caution if discontinuing an alpha2-agonist in a patient receiving a TCA. Consider therapy modification
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
Methyldopa interferes with the following laboratory tests: urinary uric acid (phosphotungstate method), serum creatinine (alkaline picrate method), AST (colorimetric method), and urinary catecholamines (falsely high levels)
May lead to false-positive aldosterone/renin ratio (ARR) (Funder 2016)
Adverse Reactions
Frequency not defined:
Cardiovascular: Bradycardia, cardiac failure, exacerbation of angina pectoris, myocarditis, orthostatic hypotension, paradoxical pressor response (intravenous use), pericarditis, peripheral edema, prolonged carotid sinus syncope, vasculitis
Central nervous system: Bell’s palsy, cerebrovascular insufficiency (symptoms), choreoathetosis, decreased mental acuity, depression, dizziness, drug fever, headache, nightmares, paresthesia, Parkinson’s disease, sedation
Dermatologic: Skin rash, toxic epidermal necrolysis
Endocrine & metabolic: Amenorrhea, decreased libido, gynecomastia, hyperprolactinemia, weight gain
Gastrointestinal: Abdominal distention, colitis, constipation, diarrhea, flatulence, glossalgia, melanoglossia, nausea, pancreatitis, sialadenitis, vomiting, xerostomia
Genitourinary: Breast hypertrophy, impotence, lactation
Hematologic & oncologic: Bone marrow depression, eosinophilia, granulocytopenia, hemolytic anemia, leukopenia, positive ANA titer, positive direct Coombs test, thrombocytopenia
Hepatic: Abnormal hepatic function tests, hepatic disease (hepatitis), jaundice
Neuromuscular & skeletal: Arthralgia, lupus-like syndrome, myalgia, positive rheumatoid factor, weakness
Renal: Increased blood urea nitrogen
Respiratory: Nasal congestion
Miscellaneous: Positive LE cell preparation
Warnings/Precautions
Concerns related to adverse effects:
- Edema: May produce clinical edema or weight gain; discontinue if edema worsens or signs of heart failure arise. Mild edema may be controlled with the concomitant use of diuretic therapy.
- Hematologic effects: Rare cases of reversible granulocytopenia and thrombocytopenia have been reported. May rarely produce hemolytic anemia; positive Coombs test occurs in 10% to 20% of patients usually occurring between 6 and 12 months of therapy; perform complete blood count (CBC) periodically. If methyldopa-induced Coombs-positive hemolytic anemia occurs during therapy, discontinue use and do not reinitiate; Coombs test may not revert back to normal for weeks to months following discontinuation.
- Hepatic effects: May rarely produce hepatic disorders including fatal hepatic necrosis. Discontinue use and do not reinitiate if fever, abnormal liver function tests, or jaundice is present.
- Sedation: Usually transient, sedation may occur with initiation or whenever the dose is increased.
Disease-related concerns:
- Cerebrovascular disease: Patients with severe bilateral cerebrovascular disease have exhibited involuntary choreoathetotic movements (rare); discontinue use if these symptoms develop.
- Hepatic impairment: Use with caution in patients with history of hepatic disease or impairment.
- Pheochromocytoma: Not recommended in patients with pheochromocytoma.
- Renal impairment: Use with caution in patients with renal impairment; may respond to smaller doses. The active metabolites of methyldopa accumulate in patients with renal impairment.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Surgical patients: Patients on methyldopa may need less anesthetic agents (Miller 1968; Miller 2010).
Dosage form specific issues:
- Injection: Do not use injectable if bisulfite allergy.
Other warnings/precautions:
- Tolerance: May occur usually between the second and third month of therapy; adding a diuretic or increasing the dosage of methyldopa frequently restores blood pressure control.
Monitoring Parameters
Blood pressure (standing and sitting/lying down), CBC, liver enzymes (periodically during the first 6 to 12 weeks or when unexplained fever occurs), Coombs test (direct) (may obtain prior to initiation and at 6 and 12 months); blood pressure monitor required during IV administration
The 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults (ACC/AHA [Whelton 2017]):
Confirmed hypertension and known CVD or 10-year ASCVD risk ≥10%: Target blood pressure <130/80 mm Hg is recommended
Confirmed hypertension without markers of increased ASCVD risk: Target blood pressure <130/80 mm Hg may be reasonable
Pregnancy
Pregnancy Considerations
Methyldopa crosses the placenta.
Available data show use during pregnancy does not cause fetal harm and improves fetal outcomes.
Chronic maternal hypertension may increase the risk of birth defects, low birth weight, preterm delivery, stillbirth, and neonatal death. Actual fetal/neonatal risks may be related to duration and severity of maternal hypertension. Untreated hypertension may also increase the risks of adverse maternal outcomes, including gestational diabetes, myocardial infarction, preeclampsia, stroke, and delivery complications (ACOG 203 2019).
If treatment for chronic hypertension during pregnancy is needed, oral methyldopa is an option; however, other agents may be preferred due to adverse events and decreased effectiveness when compared to other medications (ACOG 203 2019). Females with preexisting hypertension may continue their medication during pregnancy unless contraindications exist (Regitz-Zagrosek [ESC 2018]).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience fatigue or headache. Have patient report immediately to prescriber signs of liver problems (dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin), signs of infection, severe dizziness, passing out, severe loss of strength and energy, bruising, bleeding, shortness of breath, excessive weight gain, swelling of arms or legs, or abnormal movements (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.