Boxed Warning
Risks with neuroaxial administration (Infumorph, Duramorph, Mitigo):
Because of the risk of severe adverse effects when the epidural or intrathecal route of administration is employed, patients must be observed in a fully equipped and staffed environment for at least 24 hours after the initial dose. Single-dose Duramorph neuraxial administration may result in acute or delayed respiratory depression for up to 24 hours. Monitor patients receiving Infumorph or Mitigo, as appropriate, for the first several days after catheter implantation.
Ethanol use (extended-release capsules):
Instruct patients not to consume alcoholic beverages or use prescription or nonprescription products that contain alcohol while taking morphine extended-release (ER) capsules. The coingestion of alcohol with morphine ER may result in increased plasma levels and a potentially fatal overdose of morphine.
Addiction, abuse, and misuse:
Morphine exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient's risk prior to prescribing morphine and monitor all patients regularly for the development of these behaviors and conditions.
Opioid analgesic Risk Evaluation and Mitigation Strategy (REMS):
To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the FDA has required a REMS for these products. Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS compliant education programs available to health care providers. Health care providers are strongly encouraged to complete a REMS-compliant education program; counsel patients and/or their caregivers, with every prescription, on safe use, serious risks, storage, and disposal of these products; emphasize to patients and their caregivers the importance of reading the Medication Guide every time it is provided by their pharmacist; and consider other tools to improve patient, household, and community safety.
Life-threatening respiratory depression:
Serious, life-threatening, or fatal respiratory depression may occur with use of morphine. Monitor for respiratory depression, especially during initiation of morphine or following a dose increase. Swallow morphine ER formulations whole. ER capsule contents may be sprinkled on applesauce and swallowed immediately without chewing. Crushing, chewing, or dissolving the tablets or contents within the capsule can cause rapid release and absorption of a potentially fatal dose of morphine. Because of delay in maximum CNS effect with IV administration (30 minutes), rapid IV administration may result in overdosing. Observe patients in a fully equipped and staffed environment for at least 24 hours after each test dose of Infumorph or Mitigo and, as indicated, for the first several days after surgery.
Neonatal opioid withdrawal syndrome:
Prolonged use of morphine during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available.
Accidental ingestion:
Accidental ingestion of even one dose of morphine, especially by children, can result in a fatal overdose of morphine.
Risk of medication errors (oral solution):
Ensure accuracy when prescribing, dispensing, and administering morphine oral solution. Dosing errors due to confusion between mg and mL, and other morphine solutions of different concentrations, can result in accidental overdose and death
Risks from concomitant use with benzodiazepines or other CNS depressants:
Concomitant use of opioids with benzodiazepines or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of morphine and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Capsule Extended Release 24 Hour, Oral, as sulfate:
Kadian: 10 mg [contains brilliant blue fcf (fd&c blue #1)]
Kadian: 10 mg [contains brilliant blue fcf (fd&c blue #1), corn starch]
Kadian: 20 mg [contains corn starch, fd&c yellow #10 (quinoline yellow)]
Kadian: 20 mg [DSC] [contains fd&c yellow #10 (quinoline yellow)]
Kadian: 30 mg [DSC] [contains brilliant blue fcf (fd&c blue #1)]
Kadian: 30 mg [contains brilliant blue fcf (fd&c blue #1), corn starch]
Kadian: 40 mg [contains brilliant blue fcf (fd&c blue #1), corn starch, fd&c yellow #10 (quinoline yellow)]
Kadian: 40 mg [DSC] [contains brilliant blue fcf (fd&c blue #1), fd&c yellow #10 (quinoline yellow)]
Kadian: 50 mg [contains brilliant blue fcf (fd&c blue #1), corn starch, fd&c red #40]
Kadian: 50 mg [DSC] [contains brilliant blue fcf (fd&c blue #1), fd&c red #40]
Kadian: 60 mg [contains brilliant blue fcf (fd&c blue #1), corn starch, fd&c red #40]
Kadian: 60 mg [DSC] [contains brilliant blue fcf (fd&c blue #1), fd&c red #40]
Kadian: 80 mg [contains brilliant blue fcf (fd&c blue #1), corn starch, fd&c red #40, fd&c yellow #6 (sunset yellow)]
Kadian: 80 mg [DSC] [contains brilliant blue fcf (fd&c blue #1), fd&c red #40, fd&c yellow #6 (sunset yellow)]
Kadian: 100 mg [contains brilliant blue fcf (fd&c blue #1), corn starch, fd&c yellow #10 (quinoline yellow)]
Kadian: 100 mg [DSC] [contains brilliant blue fcf (fd&c blue #1), fd&c yellow #10 (quinoline yellow)]
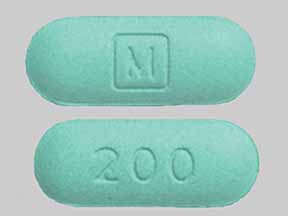
Kadian: 200 mg [DSC]
Kadian: 200 mg [contains corn starch]
Generic: 10 mg, 20 mg, 30 mg, 40 mg, 45 mg, 50 mg, 60 mg, 75 mg, 80 mg, 90 mg, 100 mg, 120 mg
Device, Intramuscular, as sulfate:
Generic: 10 mg/0.7 mL (0.7 mL)
Solution, Injection, as sulfate:
Generic: 8 mg/mL (1 mL [DSC]); 15 mg/mL (1 mL [DSC])
Solution, Injection, as sulfate [preservative free]:
Duramorph: 0.5 mg/mL (10 mL); 1 mg/mL (10 mL) [antioxidant free]
Infumorph 200: 200 mg/20 mL (10 mg/mL) (20 mL) [antioxidant free]
Infumorph 500: 500 mg/20 mL (25 mg/mL) (20 mL) [antioxidant free]
Mitigo: 200 mg/20 mL (10 mg/mL) (20 mL); 500 mg/20 mL (25 mg/mL) (20 mL)
Generic: 0.5 mg/mL (10 mL); 1 mg/mL (10 mL); 2 mg/mL (1 mL); 4 mg/mL (1 mL); 5 mg/mL (1 mL); 8 mg/mL (1 mL); 10 mg/mL (1 mL)
Solution, Intravenous, as sulfate:
Generic: 1 mg/mL (30 mL); 25 mg/mL (4 mL [DSC], 10 mL [DSC]); 50 mg/mL (20 mL, 50 mL)
Solution, Intravenous, as sulfate [preservative free]:
Generic: 1 mg/mL (30 mL); 2 mg/mL (1 mL); 4 mg/mL (1 mL); 150 mg/30 mL (30 mL [DSC]); 8 mg/mL (1 mL); 10 mg/mL (1 mL); 15 mg/mL (1 mL [DSC]); 25 mg/mL (10 mL)
Solution, Oral, as sulfate:
Generic: 10 mg/5 mL (5 mL, 15 mL, 100 mL, 500 mL); 20 mg/5 mL (5 mL, 100 mL, 500 mL); 20 mg/mL (30 mL, 118 mL, 120 mL); 100 mg/5 mL (15 mL, 30 mL, 120 mL, 240 mL)
Suppository, Rectal, as sulfate:
Generic: 5 mg (12 ea); 10 mg (12 ea); 20 mg (12 ea); 30 mg (12 ea)
Tablet, Oral, as sulfate:
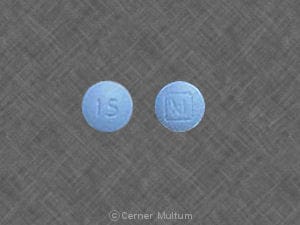
Generic: 15 mg, 30 mg
Tablet ER 12 Hour Abuse-Deterrent, Oral, as sulfate:
MorphaBond ER: 15 mg [contains brilliant blue fcf (fd&c blue #1), fd&c red #40, fd&c yellow #6 (sunset yellow)]
MorphaBond ER: 30 mg [contains fd&c blue #2 (indigotine), fd&c red #40]
MorphaBond ER: 60 mg [contains fd&c red #40, fd&c yellow #6 (sunset yellow)]
MorphaBond ER: 100 mg [contains fd&c blue #2 (indigotine), fd&c red #40, fd&c yellow #6 (sunset yellow)]
Tablet Extended Release, Oral, as sulfate:
MS Contin: 15 mg [contains fd&c blue #2 (indigotine)]
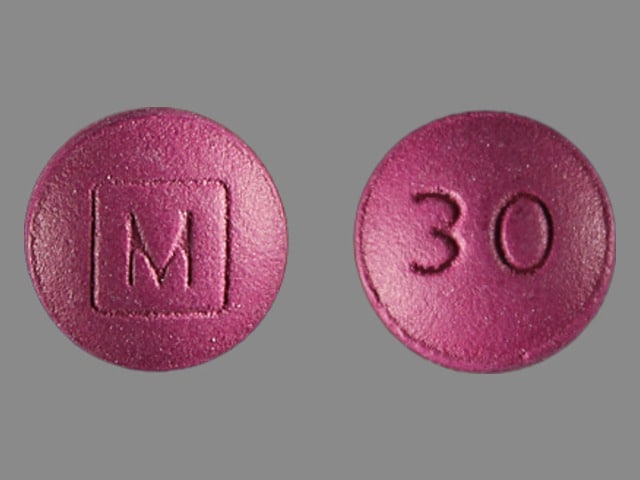
MS Contin: 30 mg [contains brilliant blue fcf (fd&c blue #1)]
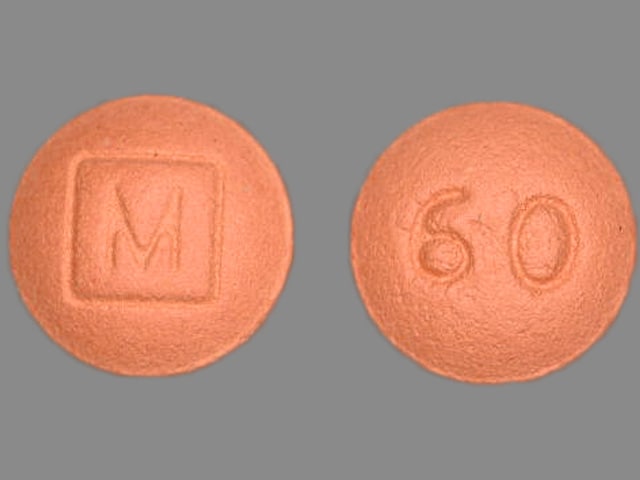
MS Contin: 60 mg [contains fd&c yellow #10 (quinoline yellow)]
MS Contin: 100 mg
MS Contin: 200 mg [contains brilliant blue fcf (fd&c blue #1), fd&c yellow #10 (quinoline yellow)]
Generic: 15 mg, 30 mg, 60 mg, 100 mg, 200 mg
Tablet Extended Release Abuse-Deterrent, Oral, as sulfate:
Arymo ER: 15 mg (100 ea); 30 mg (100 ea) [contains fd&c blue #2 (indigotine)]
Arymo ER: 60 mg (100 ea)
Pharmacology
Mechanism of Action
Binds to opioid receptors in the CNS, causing inhibition of ascending pain pathways, altering the perception of and response to pain; produces generalized CNS depression
Pharmacokinetics/Pharmacodynamics
Absorption
Oral: Variable
Distribution
Distributes to skeletal muscle, liver, kidneys, lungs, intestinal tract, spleen and brain; Vd:
Cancer patients (Children age 1.7 to 18.7 years): Median: 5.2 L/kg; a significantly higher Vd was observed in children <11 years (median: 7.1 L/kg) versus >11 years (median: 4.7 L/kg) (Hunt 1999)
Adults: 1 to 6 L/kg; binds to opioid receptors in the CNS and periphery (eg, GI tract)
Metabolism
Hepatic via conjugation with glucuronic acid primarily to morphine-6-glucuronide (M6G) (active analgesic) and morphine-3-glucuronide (M3G) (inactive as analgesic; may contribute to CNS stimulation [Lugo 2002]); minor metabolites include morphine-3-6-diglucuronide; other minor metabolites include normorphine (active) and morphine 3-ethereal sulfate
Excretion
Urine (primarily as morphine-3-glucuronide, neonates: 3% to 15%; adults: ~2% to 12% excreted unchanged); feces (~7% to 10%). It has been suggested that accumulation of morphine-6-glucuronide might cause toxicity with renal insufficiency. All of the metabolites (ie, morphine-3-glucuronide, morphine-6-glucuronide, and normorphine) have been suggested as possible causes of neurotoxicity (eg, myoclonus).
Clearance: Note: In pediatric patients, adult values are reached by 6 months to 2.5 years of age (McRorie 1992)
Preterm: 0.5 to 3 mL/minute/kg
Neonates 1 to 7 days: Median: 5.5 mL/minute/kg (range: 3.2 to 8.4 mL/minute/kg) (McRorie 1992)
Neonates 8 to 30 days: Median: 7.4 mL/minute/kg (range: 3.4 to 13.8 mL/minute/kg) (McRorie 1992)
Infants 1 to 3 months: Median: 10.5 mL/minute/kg (range: 9.8 to 20.1 mL/minute/kg) (McRorie 1992)
Infants 3 to 6 months: Median: 13.9 mL/minute/kg (range: 8.3 to 24.1 mL/minute/kg) (McRorie 1992)
Infants 6 months to Children 2.5 years: Median: 21.7 mL/minute/kg (range: 5.8 to 28.6 mL/minute/kg) (McRorie 1992)
Preschool Children: 20 to 40 mL/minute/kg
Cancer patients (Children age: 1.7 to 18.7 years): Median: 23.1 mL/minute/kg; a significantly higher clearance was observed in children <11 years (median: 37.4 mL/minute/kg) versus >11 years (median: 21.9 mL/minute/kg) (Hunt 1999)
Children with sickle cell disease (age: 6 to 19 years): ~36 mL/minute/kg (range: 6 to 59 mL/minute/kg) (Dampier 1995)
Adults: 20 to 30 mL/minute/kg
Onset of Action
Patient dependent; dosing must be individualized: Oral (immediate release): ~30 minutes; IV: 5 to 10 minutes
Time to Peak
Plasma:
Tablets, oral solution, epidural: 1 hour
Extended release tablets: 3 to 4 hours; Kadian: ~10 hours
Suppository: 20 to 60 minutes
SubQ: 50 to 90 minutes
IM: 30 to 60 minutes
IV: 20 minutes
Cerebrospinal fluid: After an oral dose of controlled release morphine concentrations peak at 8 hours for both normal and reduced renal function; morphine-6-glucuronide (active analgesic) and morphine-3-glucuronide distribution into the CNS may be delayed peaking at 12 hours in patients with normal renal function or up to 24 hours in patients with ESRD (peak level of morphine-6-glucuronide is ~15 times higher than patients with normal renal function) (D'Honneur 1994).
Duration of Action
Patient dependent; dosing must be individualized: Pain relief:
Immediate-release formulations (tablet, oral solution, injection): 3 to 5 hours
Extended-release capsule and tablet: 8 to 24 hours (formulation dependent)
Epidural or intrathecal: Single dose: Up to 24 hours (Bujedo 2012)
Suppository: 3 to 7 hours
Half-Life Elimination
Preterm: 10 to 20 hours
Neonates: 7.6 hours (range: 4.5 to 13.3 hours)
Infants 1 to 3 months: Median: 6.2 hours (range: 5 to 10 hours) (McRorie 1992)
Infants 3 to 6 months: Median: 4.5 hours (range: 3.8 to 7.3 hours) (McRorie 1992)
Infants 6 months to Children 2.5 years: Median: 2.9 hours (range: 1.4 to 7.8 hours) (McRorie 1992)
Preschool Children: 1 to 2 hours
Children with sickle cell disease (age: 6 to 19 years): ~1.3 hours (Dampier 1995)
Adults: Immediate-release forms: 2 to 4 hours; Kadian: 11 to 13 hours
Protein Binding
Premature Infants: <20%; Adults: 20% to 35%
Use: Labeled Indications
Pain management, acute and chronic pain:
Injection: Management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
Preservative-free solutions only:
Duramorph: Epidural or intrathecal management of pain without attendant loss of motor, sensory, or sympathetic function. Note: Not for use in continuous microinfusion devices.
Infumorph, Mitigo: Used in continuous microinfusion devices for intrathecal or epidural administration in management of intractable chronic pain severe enough to require an opioid analgesic and for which less invasive means of controlling pain are inadequate. Note: Not for single-dose IV, IM, or subcutaneous administration or single-dose neuraxial injection.
Oral:
Extended release: Management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.
Immediate release: Management of acute and chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. Oral solution 100 mg per 5 mL (20 mg/mL) is for opioid-tolerant patients.
Rectal: Management of acute and chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
Limitations of use: Reserve morphine for use in patients for whom alternative treatment options (eg, nonopioid analgesics, opioid combination products) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain. ER formulations are not indicated as as-needed analgesics.
Use: Off Label
Critically ill patients in the ICU (analgesia and sedation)yes
Based on the Society of Critical Care Medicine guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU, morphine is an effective and recommended agent for analgesia and sedation in critically ill patients.
Dyspnea in palliative care patientsyes
Based on Cancer Care Ontario's symptom management guide-to-practice: dyspnea, morphine is an effective and recommended agent for the management of dyspnea in palliative care patients.
Contraindications
Hypersensitivity (eg, anaphylaxis) to morphine or any component of the formulation; significant respiratory depression; acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment; concurrent use of monoamine oxidase inhibitors (MAOIs) or use of MAOIs within the last 14 days; GI obstruction, including paralytic ileus (known or suspected).
Documentation of allergenic cross-reactivity for opioids is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Additional contraindications:
Epidural/intrathecal: Infection at infusion site; concomitant anticoagulant therapy; uncontrolled bleeding diathesis; presence of any other concomitant therapy or medical condition that would render administration hazardous; upper airway obstruction.
Canadian labeling: Additional contraindications (not in US labeling):
Contraindications may vary per product labeling; refer also to product labels: Suspected surgical abdomen (eg, acute appendicitis, pancreatitis); disease/condition that affects bowel transit (known or suspected); chronic obstructive airway; status asthmaticus; management of acute pain, including use in outpatient or day surgeries; mild, intermittent, or short-duration pain that can be managed with other pain medications; acute respiratory depression; hypercarbia; cor pulmonale; acute alcoholism; delirium tremens; convulsive disorders; severe CNS depression; increased cerebrospinal; intracranial pressure; brain tumor; head injury; cardiac arrhythmias; pregnancy; use during labor and delivery; breastfeeding; alcohol consumption or medications containing alcohol (Kadian, M-Eslon product monographs); toxic psychosis and severe kyphoscoliosis (Kadian product monograph); severe cirrhosis (M-Ediat product monograph); hypotension (M-Eslon product monograph), emotional instability and/or suicidal ideation (Statex product monograph); surgical anastomosis (morphine sulfate inj Sandoz product monograph)
Dosage and Administration
Dosing: Adult
Pain management, moderate to severe pain: Note: Opioids may be part of a comprehensive, multimodal, patient-specific treatment plan for pain. Maximize nonopioid analgesia, if appropriate, prior to initiation of opioid analgesia (CDC [Dowell 2016]; Hill 2018). Dosing provided is based on typical doses and some patients may require higher or lower doses. Individualize dosing and dosing intervals based on patient-specific factors (eg, comorbidities, severity of pain, concomitant medications, general condition, degree of opioid experience/tolerance) and titrate to patient-specific treatment goals (eg, improvement in function and quality of life, decrease in pain using a validated pain rating scale). Use the lowest effective dose for the shortest period of time. For acute non-cancer-related pain severe enough to require an opioid, utilize multimodal pain control, maximize nonopioid analgesics, and limit the quantity prescribed to the expected duration of acute pain; a quantity sufficient for ≤3 days is often adequate, whereas >7 days is rarely needed. Do not use long-acting preparations for treatment of acute pain in opioid-naive patients (APS 2016; CDC [Dowell 2016]). Before starting opioid therapy for chronic pain, establish realistic goals for pain and function, and consider how therapy will be discontinued if benefits do not outweigh risks (CDC [Dowell 2016]).
Acute pain:
General dosing: Note: Dosing presented in this section is for opioid-naive patients. Patients who are opioid-tolerant will likely require higher dosing; adjust doses accordingly (Arnold 2019).
Oral: Opioid-naive patients:
Immediate release: Oral solution, Tablet:
Note: Consider the use of other more commonly prescribed oral opioids (eg, oxycodone) instead of morphine (Pino 2019). The 100 mg/5 mL (or 20 mg/mL) concentrated oral solution is not intended for opioid-naive patients.
Initial: 10 mg every 4 hours as needed; if pain is not relieved, may increase dose as tolerated. May give up to 30 mg every 4 hours as needed for severe, acute pain in hospitalized patients at low risk for respiratory depression (APS 2016; Herzig 2019; Pharmacist’s Letter [Cupp 2012]; manufacturer's labeling).
IV: Opioid-naive patients:
Intermittent: Initial: 1 to 4 mg every 1 to 4 hours as needed; if pain is not relieved, may increase dose as tolerated. May give up to 10 mg every 4 hours as needed for severe, acute pain in hospitalized patients at low risk for respiratory depression (APS 2016; Herzig 2019; Mariano 2019; SCCM [Barr 2013]; manufacturer's labeling). For some severe acute pain episodes (eg, trauma), may initially give more frequently (eg, every 5 to 15 minutes) if needed and titrate to pain relief; once pain relief is achieved, reduce frequency (eg, every 3 to 4 hours as needed) (Lvovschi 2008; Patanwala 2010). Note: When IV access is not available, SubQ administration using similar dosing may be considered; however, repeated intermittent SubQ injections cause local tissue irritation, pain, and induration and are not recommended (Mariano 2019).
Patient-controlled analgesia (Mariano 2019):
|
Example IV Patient-Controlled Analgesia Initial Dose Ranges for Opioid-Naive Patientsa |
|
|---|---|
|
aFor use to maintain pain control after initial pain control achieved. May adjust dosing and provide rescue bolus doses (eg, 0.5 to 2.5 mg) if analgesia is inadequate (Mariano 2019). bThe use of a continuous background infusion for patient-controlled analgesia is generally not recommended for most patients because of the risk of respiratory depression, and use should be limited to carefully selected patients who are opioid tolerant and/or receiving care in a critical care unit, or if required to maintain baseline opioid dosing during intervals when oral or transdermal opioid administration is not possible (Arnold 2019; Mariano 2019). |
|
|
Usual concentration |
1 mg/mL |
|
Demand dose |
Usual range: 0.5 to 2.5 mg |
|
Basal dose |
In general, a continuous (basal) infusion is not recommended in an opioid-naive patient (ISMP 2009)b |
|
Lockout interval |
5 to 10 minutes |
|
Maximum cumulative dose |
7.5 mg within 1 hour (or 30 mg within a 4-hour period) |
IM (not recommended for routine use): Opioid-naive patients: Initial: 5 to 10 mg every 3 to 4 hours as needed; if pain is not relieved, may increase dose as tolerated. Note: IM administration is generally not recommended due to pain associated with injection, variable absorption, and delayed time to peak effect (APS 2016; Mariano 2019).
Acute pain (specific indications):
Acute coronary syndrome, refractory ischemic chest pain:
Note: Use only in patients with continued ischemic chest pain despite maximally tolerated anti-ischemic medications (ACC/AHA [Amsterdam 2014]). Routine use in patients with acute coronary syndrome has been associated with worse clinical outcomes and concomitant use with oral P2Y12 inhibitors may diminish antiplatelet effects (Duarte 2019; Kubica 2016; Meine 2005).
IV: 2 to 4 mg initially, followed by 2 to 8 mg every 5 to 15 minutes as needed (ACCF/AHA [O'Gara 2013]; Reeder 2019; Simons 2019) or 1 to 5 mg initially, followed by 1 to 5 mg every 5 to 30 minutes as needed (ACC/AHA [Amsterdam 2014]).
Acute pain (eg, breakthrough cancer pain) in patients on chronic opioid therapy for pain: Oral, IV, SubQ: Usual dose: In conjunction with the scheduled long-acting opioid, administer 5% to 20% of the basal daily morphine milligram equivalents (MME) requirement given as needed using an IR formulation with subsequent dosage adjustments based upon response (Arnold 2019; Azhar 2019; Portenoy 2019).
Critically ill patients in the ICU (analgesia and sedation) (off-label use):
Note: Multimodal approaches (eg, a combination of analgesics and techniques) should typically be employed for pain control in this setting. Pain should be monitored using validated scales (eg, behavioral pain scale, critical-care observation tool) in medical, postoperative, or trauma (excluding brain injury) ICU patients who are unable to self-report (SCCM [Devlin 2018]).
IV:
Loading dose: 2 to 10 mg, followed by maintenance dosing (Tietze 2019). Note: More than 1 loading dose may be needed; onset of action following IV administration is 5 to 10 minutes. Reduce or omit initial loading dose in select patients (eg, older, hypovolemic, at-risk for hemodynamic compromise) (Tietze 2019).
Maintenance dosing: 2 to 4 mg every 1 to 2 hours or 4 to 8 mg every 3 to 4 hours (SCCM [Barr 2013]; Tietze 2019).
Postoperative pain:
Initial pain control in the post-anesthesia care unit: IV: 1 to 3 mg given as frequently as every 5 minutes until adequate pain relief or unwanted side effects (eg, respiratory depression, oxygen desaturation, hypotension) occur. Note: A maximum cumulative dose (eg, 20 mg) prompting reevaluation of continued morphine use and/or dose should be included as part of any medication order intended for short-term use (eg, post-anesthesia care unit orders); refer to institution-specific protocols as appropriate (Aubrun 2012; Casserly 2019; Mariano 2019).
Ongoing pain control: IV: 1 to 4 mg every 1 to 4 hours as needed; may give up to 10 mg every 2 to 4 hours as needed for severe, acute pain in patients at low risk for respiratory depression (APS 2016; Casserly 2019; Mariano 2019; SCCM [Barr 2013]). If patient-controlled analgesia is needed, refer to Example IV Patient-Controlled Analgesia Initial Dose Ranges for Opioid-Naive Patients table.
Sickle cell disease, vaso-occlusive pain:
Note: Dosing presented is for patients in emergency department and hospital settings (including day hospitals) whose previous opioid dose for prior episodes is unknown or who rarely require opioids for pain management. If opioid dose given for a prior episode is known, choose initial dose based on intensity of pain in comparison with previous episode and previous effective dose (DeBaun 2019).
IV: Initial: 0.1 to 0.15 mg/kg (maximum initial dose: 10 mg) given once within 30 minutes of presentation, reassess pain within 20 minutes; if continued severe pain, may repeat with doses of 0.02 to 0.05 mg/kg every 20 to 30 minutes to achieve pain relief (DeBaun 2019). If IV access is difficult, may administer SubQ (NIH 2014). If pain relief is not achieved after ≥3 doses, hospitalization for around-the-clock parenteral analgesics is generally indicated. Evaluate need for long-acting opioid; if patient usually requires a long-acting opioid at home, may convert to oral home regimen once IV dose is roughly equivalent to long-acting opioid dose (DeBaun 2019).
Chronic pain, including chronic cancer pain:
Note: Opioids, including morphine, are not the preferred therapy for chronic noncancer pain due to insufficient evidence of benefit and risk of serious harm; nonpharmacologic treatment and nonopioid analgesics are preferred, with the exception of chronic pain from sickle cell disease and end-of-life care. Opioids, including morphine, should only be considered in patients who experience clinically meaningful improvement in pain and function that outweighs patient safety risks (CDC [Dowell 2016; Dowell 2019]).
Opioid-naive patients: In general, for noncancer pain, morphine requirement should be established using IR formulations (CDC [Dowell 2016]). With cancer pain, may switch to a long-acting formulation earlier in the course of therapy (Portenoy 2019).
Oral: Immediate release: Oral solution, Tablet: Note: The 100 mg/5 mL (or 20 mg/mL) concentrated oral solution is not intended for opioid-naive patients.
Noncancer or cancer pain: Initial: 5 to 30 mg every 4 hours as needed or scheduled around the clock for some patients (eg, cancer pain) (Paice 2011; Pharmacist’s Letter [Cupp 2012]; Portenoy 2019; Rosenquist 2019). For chronic noncancer pain, most patients will have pain control with initial doses <50 mg/day (Busse 2017).
Titration: For chronic noncancer pain, may increase the dose slowly in increments of no more than 25% to 50% of the total daily dose (Rosenquist 2019). For chronic cancer pain, may increase the fixed scheduled dose by 30% to 100% of the total dose taken in the prior 24-hour period, while taking into consideration the total amount of rescue medication used; if pain score decreased, continue current effective dosing (Paice 2011; Portenoy 2019). Note: In order to reduce risk of overdose, use caution when increasing opioid dosage to ≥50 MME/day and avoid increasing dosage to ≥90 MME/day or carefully justify a decision to titrate dosage to ≥90 MME/day (CDC [Dowell 2016]).
IV, SubQ: Note: Typically reserved for acute exacerbations or those who cannot tolerate oral administration. For progressive illnesses (eg, cancer), a continuous IV or SubQ infusion, with or without a patient-controlled analgesia option, can also be used as pain requirements increase.
Noncancer or cancer pain: IV: Initial: 2 to 5 mg every 2 to 4 hours as needed (Portenoy 2019).
Cancer pain or palliative care: SubQ: Initial: 2 to 5 mg every 3 to 4 hours as needed. If a continuous SubQ infusion is employed, to maintain comfort, the SubQ infusion rate should generally not exceed 5 mL/hour (Portenoy 2019).
Opioid-tolerant patients (also refer to the section Dose conversions for pain management):
Oral: Extended release:
Note: Although manufacturer's labeling contains directions for initiating ER morphine products in opioid-naive patients with chronic pain, these preparations should not be used as initial therapy. Instead, treatment should be initiated with an IR preparation to more accurately determine the daily opioid requirement and decrease the risk of overdose. Unless pain is associated with cancer, palliative care, or sickle cell disease, the Centers for Disease Control and Prevention recommends that ER opioids be reserved for patients who have received IR opioids daily for ≥1 week yet continue to experience severe, continuous pain (CDC [Dowell 2016]).
Capsules, extended release (Kadian): See Dose conversions for pain management: Calculated dose may be administered once daily or in 2 equally divided doses administered every 12 hours; may consider dose reduction with first several doses when converting from IR formulations. Example initial dose: 30 mg once daily or 15 mg every 12 hours. Dose adjustments may be made as frequently as every 1 to 2 days.
Tablets, extended release (Arymo ER, MorphaBond ER, MS Contin): See Dose conversions for pain management: Calculated dose may be administered in 2 equally divided doses (every 12 hours) or 3 equally divided doses (every 8 hours); may consider dose reduction with first several doses when converting from IR formulations. Example initial dose: 15 mg every 8 or 12 hours. Dose adjustments may be made as frequently as every 1 to 2 days.
Dose conversions for pain management: Note: Equianalgesic conversions serve only as a general guide to estimate opioid dose equivalents. Multiple factors must be considered for safely individualizing conversion of opioid analgesia. In general, for noncancer pain, the decision to convert from an IR to an ER formulation should be individualized and reserved for those with severe continuous pain who have been taking opioids for ≥1 week (Rosenquist 2019).
Converting from oral morphine to parenteral morphine:
Approximate equivalency: 30 mg (oral morphine): 10 mg (IV/SubQ morphine) (Pharmacist’s Letter [Cupp 2012]).
Converting from oral IR morphine to oral ER morphine preparations:
Arymo ER, MorphaBond ER, MS Contin: Total daily oral morphine dose may be administered either in 2 divided doses (every 12 hours) or in 3 divided doses (every 8 hours).
Kadian: Total daily oral morphine dose administered once daily; in patients experiencing inadequate analgesia with once-daily dosing, total daily dose can be administered in 2 divided doses (every 12 hours).
Converting from oral morphine to rectal morphine:
Although the bioavailability of rectal morphine is believed to approximate oral morphine (ie, 1:1), absorption is variable and may be higher or lower than expected. Therefore, when switching from oral to rectal dosing, a reduction in rectal dose may be necessary (Brokjær 2015; Portenoy 2019).
Converting to/from morphine (parenteral or oral) to/from a different opioid (parenteral or oral):
Refer to published equianalgesic opioid conversion data for guidance (or refer to institutional protocols). Provided conversion ratios are only approximations and substantial interpatient variability exists; therefore, it is safer to underestimate a patient's daily oral requirement and provide breakthrough pain relief with IR formulations rather than risk overestimating daily requirements. When switching to a new opioid (except to/from methadone), reduce the initial daily calculated equianalgesic dose of the new opioid by 25% to 50% to adjust for lack of complete mu receptor cross-tolerance (conversions to/from methadone are highly variable and require extreme caution) (Portenoy 2019).
Discontinuation of pain management therapy:
When reducing the dose or discontinuing chronic opioid therapy, the dose should be gradually tapered. An optimal tapering schedule has not been established (CDC [Dowell 2016]). Proposed schedules range from slow (eg, 10% reduction per week) to rapid (eg, 25% to 50% reduction every few days) (CDC 2015). Individualize to minimize withdrawal while considering patient-specific goals and concerns as well as the opioid’s pharmacokinetics. Slower tapers may be appropriate after long-term use (eg, years), particularly in the final stage of tapering, whereas more rapid tapers may be appropriate in patients experiencing severe adverse effects (CDC [Dowell 2016]). Monitor carefully for signs/symptoms of withdrawal. If the patient displays withdrawal symptoms, consider slowing the taper schedule; alterations may include increasing the interval between dose reductions, decreasing amount of daily dose reduction, pausing the taper and restarting when the patient is ready, and/or coadministration of an alpha-2 agonist (eg, clonidine) to blunt withdrawal symptoms (Berna 2015; CDC [Dowell 2016]). Continue to offer nonopioid analgesics as needed for pain management during the taper; consider nonopioid adjunctive treatments for withdrawal symptoms (eg, GI complaints, muscle spasm) as needed (Berna 2015; Sevarino 2019).
Neuraxial analgesia:
Epidural:
Note: Reserve use for severe pain (eg, after surgery, cancer pain). Must be administered by health care providers skilled in the care of patients receiving intraspinal opioids (APS [Chou 2016]). Use a preservative-free (PF) formulation intended for neuraxial use.
Single dose (using 0.5 or 1 mg/mL PF solution): Opioid-naive patients: Usual range: 2 to 3.75 mg (may depend upon patient comorbidities) (Bujedo 2012; Lanz 1985; Mariano 2019; Palmer 2000).
Continuous infusion (using 0.5 or 1 mg/mL PF solution): Opioid-naive patients: 0.2 to 0.4 mg/hour (Bujedo 2012). May be given alone or usually in combination with local anesthetics (eg, bupivacaine, ropivacaine); when combined with a local anesthetic, analgesic effect is increased due to synergy (Bujedo 2012; Manion 2011).
Continuous microinfusion (using a device intended for continuous microinfusion): Note: Must use a 10 mg/mL or 25 mg/mL PF solution (eg, Infumorph); dilution may be required.
Initial: 3.5 to 7.5 mg over 24 hours.
Intrathecal:
Note: Reserve use for severe pain (eg, after surgery, cancer pain). Must be administered by health care providers skilled in the care of patients receiving intraspinal opioids (APS [Chou 2016]). Use a PF formulation intended for neuraxial use. Intrathecal dose is usually 1/10 (one-tenth) that of epidural dosage (APS 2016).
Single dose (using 0.5 or 1 mg/mL PF solution): Usual range: 0.1 to 0.2 mg coadministered with a local anesthetic; repeat doses are not recommended. If pain recurs within 24 hours of administration, use of an alternative route of administration is recommended (APS 2008; Mariano 2019). Note: Although product labeling recommends doses up to 1 mg, the risk of adverse effects (eg, nausea, respiratory depression) is higher with doses >0.3 mg; however, some patients with chronic intractable pain (eg, cancer pain) may require doses up to 0.5 mg (PACC [Deer 2017]; Rathmell 2005).
Continuous microinfusion (using a device intended for continuous microinfusion): Note: Must use a 10 mg/mL or 25 mg/mL PF solution (eg, Infumorph); dilution may be required.
Initial: 0.2 to 1 mg over 24 hours.
Dyspnea in palliative care patients (off-label use):
Opioid-naive patients:
Moderate dyspnea: Immediate release (may use 100 mg/5 mL [20 mg/mL] solution): Oral, Sublingual: Initial: 5 mg every 2 to 4 hours with 2.5 mg every 2 hours as needed or on an “offer, may refuse” basis (most patients will not need every dose) (Cancer Care Ontario 2010; Dudgeon 2019; Harman 2019). Note: Consider lower initial scheduled doses (eg, 2.5 mg every 2 hours) in patients who are older, frail, or with dyspnea in a setting of heart failure or chronic obstructive pulmonary disease (Harman 2019).
Severe dyspnea: SubQ, IV: Initial: 2.5 mg; if dyspnea persists and initial dose is well tolerated, may repeat every 30 to 60 minutes (SubQ) or every 15 to 30 minutes (IV). If 2 doses are well tolerated but fail to reduce dyspnea adequately, the dose may be doubled (Dudgeon 2019).
Opioid-tolerant patients: Note: Higher initial doses will likely be needed.
Moderate or severe dyspnea: Immediate release: Oral: Consider giving 10% to 15% of the basal daily opioid requirement (calculated in morphine equivalents) every 2 hours as needed or on an “offer, may refuse” basis. Consider increasing the regular daily dose by ~25% taking into consideration breakthrough doses used in the previous 24 hours (Dudgeon 2019; Harman 2019).
Severe dyspnea: SubQ, IV: Consider giving 5% of the oral basal daily opioid requirement (calculated in morphine equivalents) every 1 hour as needed or on an “offer, may refuse” basis. For breakthrough dyspnea, if already taking a parenteral opioid, may give ~10% of the current parenteral opioid daily dose (Dudgeon 2019; Harman 2019).
Dosing: Geriatric
Refer to adult dosing. Use with caution; may require reduced dosage.
Dosing: Pediatric
Doses should be titrated to appropriate effect; use lower doses in opioid naive patients; when changing routes of administration in chronically treated patients, please note that oral doses are approximately one-half as effective as parenteral dose.
Acute pain, moderate to severe: Note: Repeated SubQ administration causes local tissue irritation, pain, and induration. The use of IM injections is no longer recommended, especially for repeated administration due to painful administration, variable absorption, and lag time to peak effect; other routes are more reliable and less painful (American Pain Society 2016).
Infants ≤6 months, nonventilated: Note: Infants <3 months of age are more susceptible to respiratory depression; lower doses are recommended; consider frequent or continuous respiratory monitoring (eg, pulse oximetry) and be in a setting that permits rapid management of respiratory insufficiency (American Pain Society 2016; Berde 2002).
Oral: Oral solution (2 mg/mL or 4 mg/mL): 0.08 to 0.1 mg/kg/dose every 3 to 4 hours (American Pain Society 2008; Berde 2002).
IV or SubQ: 0.025 to 0.03 mg/kg/dose every 2 to 4 hours (American Pain Society 2008; Berde 2002).
Infants ≤6 months, ventilated: Note: Infants <3 months are more susceptible to respiratory depression. Patients should have continuous respiratory monitoring (eg, pulse oximetry) and be in a setting that permits rapid management of respiratory insufficiency.
IV; intermittent dosing: Infants ≥3 months: Initial: 0.05 mg/kg/dose every 2 to 4 hours; dosing based on experience in postoperative cardiothoracic patients (Penk 2018).
Continuous IV infusion: Initial: 0.008 to 0.02 mg/kg/hour (8 to 20 mcg/kg/hour); titrate carefully to effect (Berde 2002; Lynn 1998); reported dose range following titration: 0.015 to 0.04 mg/kg/hour (15 to 40 mcg/kg/hour); dosing based on studies in postoperative patients, most commonly following cardiac surgery (Koren 1985; Lynn 1998; Penk 2018; Valkenburg 2016). Lower initial doses have been reported in infants following cardiac surgery compared to non-cardiac surgical infants (Lynn 1998); infants with Down Syndrome have been shown to have similar morphine requirements postoperatively as patients without following cardiac surgery (Goot 2018; Valkenburg 2016).
Infants >6 months, Children, and Adolescents:
Oral: Immediate-release tablets, oral solution (2 mg/mL or 4 mg/mL):
Patient weight <50 kg: 0.2 to 0.5 mg/kg/dose every 3 to 4 hours as needed; some experts have recommended an initial dose of 0.3 mg/kg for severe pain; usual initial maximum dose: 15 to 20 mg (American Pain Society 2008; APA 2012; Berde 2002).
Patient weight ≥50 kg: 15 to 20 mg every 3 to 4 hours as needed (American Pain Society 2008; Berde 2002).
IM, IV, or SubQ; intermittent dosing:
Patient weight <50 kg: Opioid naïve: Initial: 0.05 mg/kg/dose; usual maximum initial dose: 1 to 2 mg/dose; higher doses may be required if pain not adequately controlled or if patient is opioid tolerant; usual range: 0.1 to 0.2 mg/kg/dose every 2 to 4 hours as needed; use lower doses in opioid naïve patients; usual maximum dose: Infants: 2 mg/dose; Children 1 to 6 years: 4 mg/dose; Children 7 to 12 years: 8 mg/dose; Adolescents: 10 mg/dose.
Patient weight ≥50 kg: Initial: 2 to 5 mg every 2 to 4 hours as needed; Use lower end of the dosing range in opioid naïve patients; higher doses have been recommended (5 to 8 mg every 2 to 4 hours as needed) and may be needed in tolerant patients (Berde 2002; Kliegman 2011).
Continuous IV infusion, SubQ continuous infusion: Note: Patients should have continuous respiratory monitoring (eg, pulse oximetry) and be in a setting that permits rapid management of respiratory insufficiency (American Pain Society 2016; Berde 2002).
Patient weight <50 kg: Initial: 0.01 mg/kg/hour (10 mcg/kg/hour); titrate carefully to effect; dosage range: 0.01 to 0.04 mg/kg/hour (10 to 40 mcg/kg/hour) (APA 2012; Friedrichsdorf 2007; Golianu 2000).
Patient weight ≥50 kg: 1.5 mg/hour (Berde 2002).
Conversion from intermittent IV morphine: Administer the patient's total daily IV morphine dose over 24 hours as a continuous infusion; titrate dose to appropriate effect.
Epidural: Astramorph/PF, Duramorph: Limited data available: Note: Must use preservative-free formulation:
Intermittent: Infants, Children, and Adolescents: 0.015 to 0.05 mg/kg (15 to 50 mcg/kg) (APA 2012; Henneberg 1993); a trial evaluating pain relief in pediatric patients after abdominal surgery (n=76; age: Newborn to 13 years; median age: 12 months) administered epidural morphine every 8 hours in combination with bupivacaine during the immediate postop period; most children achieved good pain relief with this regimen (Henneberg 1993). Maximum dose: 0.1 mg/kg (100 mcg/kg) or 5 mg/24 hours.
Continuous epidural infusion: Infants >6 months, Children, and Adolescents: 0.001 to 0.005 mg/kg/hour (1 to 5 mcg/kg/hour) (Suresh 2012).
Analgesia for minor procedures/sedation: Infants, Children, and Adolescents: IV: 0.05 to 0.1 mg/kg/dose; administer 5 minutes before the procedure; maximum dose: 4 mg; may repeat dose in 5 minutes if necessary (Cramton 2012; Zeltzer 1990).
Patient-controlled analgesia (PCA), opioid-naïve: Note: All patients should receive an initial loading dose of an analgesic (to attain adequate control of pain) before starting PCA for maintenance. Adjust doses, lockouts, and limits based on required loading dose, age, state of health, and presence of opioid tolerance. Use lower end of dosing range for opioid-naïve. Assess patient and pain control at regular intervals and adjust settings if needed (American Pain Society 2008): IV:
Children ≥5 years and Adolescents, weighing <50 kg: Note: PCA has been used in children as young as 5 years of age; however, clinicians need to assess children 5 to 8 years of age to determine if they are able to use the PCA device correctly (American Pain Society 2008).
Usual concentration: 1 mg/mL.
Demand dose: Usual initial: 0.02 mg/kg/dose; usual range: 0.01 to 0.03 mg/kg/dose.
Lockout: Usual initial: 5 doses/hour.
Lockout interval: Range: 6 to 8 minutes.
Usual basal rate: 0 to 0.03 mg/kg/hour.
Children ≥5 years and Adolescents, weighing ≥50 kg:
Usual concentration: 1 mg/mL.
Demand dose: Usual initial: 1 mg; usual range: 0.5 to 2.5 mg.
Lockout interval: Usual initial: 6 minutes; usual range: 5 to 10 minutes.
Chronic pain: Note: Patients taking opioids chronically may become tolerant and require doses higher than the usual dosage range to maintain the desired effect. Tolerance can be managed by appropriate dose titration. There is no optimal or maximal dose for morphine in chronic pain. The appropriate dose is one that relieves pain throughout its dosing interval without causing unmanageable side effects. Consider total daily dose, potency, prior opioid use, degree of opioid experience and tolerance, conversion from previous opioid (including opioid formulation), patient's general condition, concurrent medications, and type and severity of pain during prescribing process.
Oral: Extended-/controlled-release preparations: A patient's morphine requirement should be established using immediate-release formulations. Conversion to long acting products may be considered when chronic, continuous treatment is required. Higher dosages should be reserved for use only in opioid-tolerant patients.
Capsules, extended release (Avinza): Adolescents ≥18 years: Daily dose administered once daily (for best results, administer at same time each day).
Opioid-naive: Initial: 30 mg once daily; adjust in increments ≤30 mg daily every 4 days.
Capsules, extended release (Kadian): Adolescents ≥18 years: Note: Not intended for use as an initial opioid in the management of pain; use immediate release formulations before initiation. Total daily oral morphine dose may be either administered once daily or in 2 divided doses daily (every 12 hours). The first dose of Kadian may be taken with the last dose of the immediate release morphine.
Tablets, controlled release (MS Contin): Children and Adolescent able to swallow tablets whole: Usually not used as an initial opioid in the management of pain; use immediate release formulations to titrate dose. Total daily morphine dose may be administered in 2 divided doses daily (every 12 hours) or in 3 divided doses daily (every 8 hours).
Weight-directed dosing: 0.3 to 0.6 mg/kg/dose every 12 hours (Berde 1990).
Alternate dosing; fixed dosing (Berde 2002):
Patient weight 20 to <35 kg: 10 to 15 mg every 8 to 12 hours; Note: 10 mg strength not available in US.
Patient weight 35 to <50 kg: 15 to 30 mg every 8 to 12 hours.
Patient weight ≥50 kg: 30 to 45 mg every 8 to 12 hours.
Discontinuation of extended-release formulations: In general, gradually titrate dose downward (eg, every 2 to 4 days). Do not discontinue abruptly.
Conversion from other oral morphine formulations to extended-release formulations:
Avinza: Adolescents ≥18 years: Total daily morphine dose administered once daily. The first dose of Avinza may be taken with the last dose of the immediate-release morphine. Maximum daily dose: 1,600 mg/day due to fumaric acid content.
Kadian: Adolescents ≥18 years: Total daily oral morphine dose may be either administered once daily or in 2 divided doses daily (every 12 hours).
MS Contin: Children and Adolescents: Total daily oral morphine dose may be administered either in 2 divided doses daily (every 12 hours) or in 3 divided doses (every 8 hours).
Conversion from parenteral morphine or other opioids to controlled/extended release formulations: Substantial interpatient variability exists in relative potency. Therefore, it is safer to underestimate a patient's daily oral morphine requirement and provide breakthrough pain relief with immediate-release morphine than to overestimate requirements. Consider the parenteral to oral morphine ratio or other oral or parenteral opioids to oral morphine conversions.
Continuous IV infusion, SubQ continuous infusion: Children and Adolescents: 0.01 to 0.04 mg/kg/hour (10 to 40 mcg/kg/hour) (APA 2012; Friedrichsdorf 2007; Golianu 2000); opioid-tolerate patients may require higher doses; in a small study of terminal pediatric oncology patients (n=8; age range: 3 to 16 years), the median required dose was 0.04 to 0.07 mg/kg/hour (40 to 70 mcg/kg/hour); range: 0.025 to 2.6 mg/kg/hour (Miser 1980); another study evaluating subcutaneous continuous infusion in children with cancer (n=17; age range: 22 months to 22 years) had similar findings; median dose: 0.06 mg/kg/hour (60 mcg/kg/hour); range: 0.025 to 1.79 mg/kg/hour (Miser 1983).
Conversion from intermittent IV morphine: Administer the patient's total daily IV morphine dose over 24 hours as a continuous infusion; titrate dose to appropriate effect.
Sickle cell disease, acute crisis; opioid naïve patients (APS 1999; NHLBI 2014): Note: Individualize dose; titrate to effect; Infants ≥6 months, Children, and Adolescents:
Patient weight <50 kg: Initial: IV: 0.1 to 0.15 mg/kg every 2 to 4 hours; maximum dose: 7.5 mg/dose.
Patient weight ≥50 kg: Initial: IV: 5 to 10 mg every 2 to 4 hours.
Tetralogy of fallot, hypercyanotic spell (infundibular spasm): Infants and Children: Limited data available: IM, IV, SubQ: 0.1 mg/kg has been used to decrease ventilatory drive and systemic venous return (Hegenbarth 2008).
Palliative care, dyspnea management: Limited data available: Children and Adolescents:
Inhalation (nebulization; preservative-free injection): Dose should be individualized and is dependent upon patient's previous or current systemic opioid exposure; doses not intended to provide analgesic activity; current systemic analgesia should be continued: Initial dose: Equivalent to patient's 4-hour systemic morphine requirement (eg, IV or oral dose); titrate to effect (Golianu 2000); every 4 to 6 hour administration has been suggested (Cohen 2002). In the only pediatric case report (end-stage CF, age: 10 years, weight: 20 kg), an initial dose of 2.5 mg was used and final dose was 10 mg every 4 to 6 hours (Cohen 2002); from experience in adult patients, an initial dose of 5 mg has been used and reported range 2.5 to 30 mg administered up to every 4 hours (Ferraresi 2005; Shirk 2006).
Continuous IV or SubQ infusion (when oral ineffective): Initial: 0.005 mg/kg/hour (5 mcg/kg/hour); titrate for comfort (Garcia-Salido 2015); dosing based on palliative management of terminal infants with spinal muscular atrophy (type 1); intermittent IV maximum doses of 0.4 mg/kg have been reported to control symptoms of dyspnea and pain (di Pede 2018).
Oral: 0.1 mg/kg/dose every 4 hours as needed (Garcia-Salido 2015); titrate for comfort; dosing based on palliative management of terminal infants with spinal muscular atrophy (type 1); maximum doses of 0.4 mg/kg have been reported to control symptoms of dyspnea and pain (di Pede 2018).
Reconstitution
Parenteral:
IV push/intermittent infusion: May dilute to a final concentration of 0.5 to 5 mg/mL
Continuous IV infusion: Dilute in D5W, D10W, or NS to a usual final concentration of 0.1 to 1 mg/mL; more concentrated solutions may be used in patients requiring fluid restriction or high doses (Murray 2014; Sinclair-Pingel 2006); concentrations >5 mg/mL are rarely needed (Gahart 2014).
Epidural and intrathecal: Use only preservative-free injections indicated for epidural/intrathecal use. Dilution may be required (preservative-free NS recommended); determined by the individual dosage requirements and the characteristics of the continuous microinfusion device; filter through ≤5 micron microfilter before injecting into microinfusion device.
Extemporaneously Prepared
0.4 mg/mL (400 mcg/mL) Oral Solution (ASHP 2017)
A 0.4 mg/mL oral solution may be made using the 2 mg/mL oral morphine solution. Measure 10 mL (20 mg) of the 2 mg/mL oral morphine solution and transfer to a plastic amber bottle. Measure 40 mL of sterile water for irrigation and add to bottle containing the morphine. Shake to mix. Store at room temperature. Stable for 60 days.
Sauberan J, Rossi S, Kim JH. Stability of dilute oral morphine solution for neonatal abstinence syndrome. J Addict Med. 2013;7(2):113-115.23370932
Administration
Oral: Do not crush, chew, or dissolve ER formulations; swallow whole. Cutting, breaking, crushing, chewing, or dissolving ER formulations may result in uncontrolled delivery of morphine, leading to overdose and death.
Arymo ER: Swallow tablets whole, one tablet at a time, with enough water to ensure complete swallowing immediately after placing in the mouth; do not pre-soak, lick, or wet tablets prior to placing in the mouth.
Kadian: Capsules may be opened and sprinkled on applesauce and eaten immediately without chewing; do not crush, dissolve, or chew the beads, as it can result in a rapid release of a potentially fatal dose of morphine. Ensure all pellets have been swallowed by rinsing mouth. Contents of capsules may be opened and sprinkled over 10 mL water and flushed through prewetted 16F gastrostomy tube; do not administer through gastric/nasogastric tubes.
IV: Administer single-use prefilled syringes/cartridges via slow IV push over 4 to 5 minutes (rapid administration may result in chest wall rigidity). Concentrated vials are available for preparation of continuous IV infusion or PCA; refer to indication-specific infusion rates in dosing for detailed recommendations.
Epidural, intrathecal: Use only preservative-free solutions indicated for intrathecal or epidural use; refer to indication-specific infusion rates in dosing for detailed recommendations.
Rectal: Remove suppository from plastic packet and moisten suppository with water to avoid irritation. Gently insert (rounded end first) approximately a finger's length into rectum, angling it toward the umbilicus, and placing it against the rectal wall; after suppository is inserted, hold buttocks together until urge to expel ceases (Pasero 1999).
Dietary Considerations
Morphine may cause GI upset; take with food if GI upset occurs. Be consistent when taking morphine with or without meals.
Storage
Parenteral: Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Do not freeze. Store in carton until use. Protect from light. Degradation depends on pH and presence of oxygen; relatively stable in pH ≤4. Darkening of solutions indicate degradation. Do not heat-sterilize.
Oral:
Extended release: Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Protect capsules from light and moisture.
Immediate release: Store at 20°C to 25°C (68°F to 77°F). Protect from moisture.
Suppositories: Store at 20°C to 25°C (68°F to 77°F).
Morphine (Systemic) Images
Drug Interactions
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Alizapride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Alvimopan: Opioid Agonists may enhance the adverse/toxic effect of Alvimopan. This is most notable for patients receiving long-term (i.e., more than 7 days) opiates prior to alvimopan initiation. Management: Alvimopan is contraindicated in patients receiving therapeutic doses of opioids for more than 7 consecutive days immediately prior to alvimopan initiation. Consider therapy modification
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Amphetamines: May enhance the analgesic effect of Opioid Agonists. Monitor therapy
Anticholinergic Agents: May enhance the adverse/toxic effect of Opioid Agonists. Specifically, the risk for constipation and urinary retention may be increased with this combination. Monitor therapy
Antiplatelet Agents (P2Y12 Inhibitors): Morphine (Systemic) may diminish the antiplatelet effect of Antiplatelet Agents (P2Y12 Inhibitors). Morphine (Systemic) may decrease the serum concentration of Antiplatelet Agents (P2Y12 Inhibitors). Management: Consider alternative anti-ischemic/analgesic therapies (eg, beta-blockers, nitroglycerin) in patients with acute coronary syndromes treated with a P2Y12 inhibitor when possible. The risks associated with other opioids are unknown. Exceptions: Cangrelor. Consider therapy modification
Azelastine (Nasal): CNS Depressants may enhance the CNS depressant effect of Azelastine (Nasal). Avoid combination
Blonanserin: CNS Depressants may enhance the CNS depressant effect of Blonanserin. Consider therapy modification
Blood Pressure Lowering Agents: May enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Brimonidine (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromopride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Bromperidol: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Cannabis: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Chlormethiazole: May enhance the CNS depressant effect of CNS Depressants. Management: Monitor closely for evidence of excessive CNS depression. The chlormethiazole labeling states that an appropriately reduced dose should be used if such a combination must be used. Consider therapy modification
Chlorphenesin Carbamate: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
CNS Depressants: May enhance the CNS depressant effect of Opioid Agonists. Management: Avoid concomitant use of opioid agonists and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Desmopressin: Opioid Agonists may enhance the adverse/toxic effect of Desmopressin. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Dimethindene (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Diuretics: Opioid Agonists may enhance the adverse/toxic effect of Diuretics. Opioid Agonists may diminish the therapeutic effect of Diuretics. Monitor therapy
Dronabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Droperidol: May enhance the CNS depressant effect of CNS Depressants. Management: Consider dose reductions of droperidol or of other CNS agents (eg, opioids, barbiturates) with concomitant use. Exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Consider therapy modification
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
Eluxadoline: Opioid Agonists may enhance the constipating effect of Eluxadoline. Avoid combination
Erdafitinib: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Esmolol: Morphine (Systemic) may increase the serum concentration of Esmolol. Monitor therapy
Flunitrazepam: CNS Depressants may enhance the CNS depressant effect of Flunitrazepam. Consider therapy modification
Gabapentin: May enhance the CNS depressant effect of Morphine (Systemic). Morphine (Systemic) may increase the serum concentration of Gabapentin. Monitor therapy
Gastrointestinal Agents (Prokinetic): Opioid Agonists may diminish the therapeutic effect of Gastrointestinal Agents (Prokinetic). Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
HYDROcodone: CNS Depressants may enhance the CNS depressant effect of HYDROcodone. Management: Avoid concomitant use of hydrocodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Kava Kava: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
Lasmiditan: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Avoid combination
Lemborexant: May enhance the CNS depressant effect of CNS Depressants. Management: Dosage adjustments of lemborexant and of concomitant CNS depressants may be necessary when administered together because of potentially additive CNS depressant effects. Close monitoring for CNS depressant effects is necessary. Consider therapy modification
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Lofexidine: May enhance the CNS depressant effect of CNS Depressants. Management: Drugs listed as exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Monitor therapy
Lumacaftor and Ivacaftor: May decrease the serum concentration of P-glycoprotein/ABCB1 Substrates. Lumacaftor and Ivacaftor may increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Magnesium Sulfate: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Methotrimeprazine: CNS Depressants may enhance the CNS depressant effect of Methotrimeprazine. Methotrimeprazine may enhance the CNS depressant effect of CNS Depressants. Management: Reduce adult dose of CNS depressant agents by 50% with initiation of concomitant methotrimeprazine therapy. Further CNS depressant dosage adjustments should be initiated only after clinically effective methotrimeprazine dose is established. Consider therapy modification
MetyroSINE: CNS Depressants may enhance the sedative effect of MetyroSINE. Monitor therapy
Minocycline (Systemic): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Monoamine Oxidase Inhibitors: May enhance the adverse/toxic effect of Morphine (Systemic). Avoid combination
Nabilone: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nalmefene: May diminish the therapeutic effect of Opioid Agonists. Management: Avoid the concomitant use of nalmefene and opioid agonists. Discontinue nalmefene 1 week prior to any anticipated use of opioid agonistss. If combined, larger doses of opioid agonists will likely be required. Consider therapy modification
Naltrexone: May diminish the therapeutic effect of Opioid Agonists. Management: Seek therapeutic alternatives to opioids. See full drug interaction monograph for detailed recommendations. Consider therapy modification
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Opioid Agonists: CNS Depressants may enhance the CNS depressant effect of Opioid Agonists. Management: Avoid concomitant use of opioid agonists and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Opioids (Mixed Agonist / Antagonist): May diminish the analgesic effect of Opioid Agonists. Management: Seek alternatives to mixed agonist/antagonist opioids in patients receiving pure opioid agonists, and monitor for symptoms of therapeutic failure/high dose requirements (or withdrawal in opioid-dependent patients) if patients receive these combinations. Avoid combination
Orphenadrine: CNS Depressants may enhance the CNS depressant effect of Orphenadrine. Avoid combination
Oxomemazine: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
OxyCODONE: CNS Depressants may enhance the CNS depressant effect of OxyCODONE. Management: Avoid concomitant use of oxycodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Paraldehyde: CNS Depressants may enhance the CNS depressant effect of Paraldehyde. Avoid combination
Pegvisomant: Opioid Agonists may diminish the therapeutic effect of Pegvisomant. Monitor therapy
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Perampanel: May enhance the CNS depressant effect of CNS Depressants. Management: Patients taking perampanel with any other drug that has CNS depressant activities should avoid complex and high-risk activities, particularly those such as driving that require alertness and coordination, until they have experience using the combination. Consider therapy modification
P-glycoprotein/ABCB1 Inducers: May decrease the serum concentration of P-glycoprotein/ABCB1 Substrates. P-glycoprotein inducers may also further limit the distribution of p-glycoprotein substrates to specific cells/tissues/organs where p-glycoprotein is present in large amounts (e.g., brain, T-lymphocytes, testes, etc.). Monitor therapy
P-glycoprotein/ABCB1 Inhibitors: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. P-glycoprotein inhibitors may also enhance the distribution of p-glycoprotein substrates to specific cells/tissues/organs where p-glycoprotein is present in large amounts (e.g., brain, T-lymphocytes, testes, etc.). Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Piribedil: CNS Depressants may enhance the CNS depressant effect of Piribedil. Monitor therapy
Pramipexole: CNS Depressants may enhance the sedative effect of Pramipexole. Monitor therapy
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Ramosetron: Opioid Agonists may enhance the constipating effect of Ramosetron. Monitor therapy
Ranolazine: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
RifAMPin: May decrease the serum concentration of Morphine (Systemic). Monitor therapy
ROPINIRole: CNS Depressants may enhance the sedative effect of ROPINIRole. Monitor therapy
Rotigotine: CNS Depressants may enhance the sedative effect of Rotigotine. Monitor therapy
Rufinamide: May enhance the adverse/toxic effect of CNS Depressants. Specifically, sleepiness and dizziness may be enhanced. Monitor therapy
Selective Serotonin Reuptake Inhibitors: CNS Depressants may enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Specifically, the risk of psychomotor impairment may be enhanced. Monitor therapy
Serotonergic Agents (High Risk): Opioid Agonists may enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Sincalide: Drugs that Affect Gallbladder Function may diminish the therapeutic effect of Sincalide. Management: Consider discontinuing drugs that may affect gallbladder motility prior to the use of sincalide to stimulate gallbladder contraction. Consider therapy modification
Sodium Oxybate: May enhance the CNS depressant effect of CNS Depressants. Management: Consider alternatives to combined use. When combined use is needed, consider minimizing doses of one or more drugs. Use of sodium oxybate with alcohol or sedative hypnotics is contraindicated. Consider therapy modification
Succinylcholine: May enhance the bradycardic effect of Opioid Agonists. Monitor therapy
Suvorexant: CNS Depressants may enhance the CNS depressant effect of Suvorexant. Management: Dose reduction of suvorexant and/or any other CNS depressant may be necessary. Use of suvorexant with alcohol is not recommended, and the use of suvorexant with any other drug to treat insomnia is not recommended. Consider therapy modification
Tapentadol: May enhance the CNS depressant effect of CNS Depressants. Management: Avoid concomitant use of tapentadol and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Tetrahydrocannabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Tetrahydrocannabinol and Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Thalidomide: CNS Depressants may enhance the CNS depressant effect of Thalidomide. Avoid combination
Zolpidem: CNS Depressants may enhance the CNS depressant effect of Zolpidem. Management: Reduce the Intermezzo brand sublingual zolpidem adult dose to 1.75 mg for men who are also receiving other CNS depressants. No such dose change is recommended for women. Avoid use with other CNS depressants at bedtime; avoid use with alcohol. Consider therapy modification
Test Interactions
Some quinolones may produce a false-positive urine screening result for opioids using commercially-available immunoassay kits. This has been demonstrated most consistently for levofloxacin and ofloxacin, but other quinolones have shown cross-reactivity in certain assay kits. Confirmation of positive opioid screens by more specific methods should be considered.
Adverse Reactions
>10%:
Central nervous system: Drowsiness (oral: 9% to >10%), headache (<2% to >10%)
Gastrointestinal: Constipation (9% to >10%), nausea (7% to >10%), vomiting (2% to >10%)
Genitourinary: Urinary retention (<2%)
1% to 10%:
Cardiovascular: Peripheral edema (3% to 10%), chest pain (oral: 2%), atrial fibrillation (oral: <2%), bradycardia (oral, rectal: <2%), edema (oral, rectal: <2%), facial flushing (oral, rectal: <2%), flushing (oral: <2%), hypertension (oral: <2%), hypotension (oral: <2%), palpitations (oral, rectal: <2%), syncope (oral, rectal: <2%), tachycardia (oral: <2%), vasodilation (oral: <2%)
Central nervous system: Depression (oral: <2% to 10%), insomnia (oral, rectal: <2% to 10%), paresthesia (oral: <2% to 10%), dizziness (6%), anxiety (≤6%), abnormality in thinking (oral: <5%), confusion (<5%), seizure (<5%), pain (oral: 3%), abnormal dreams (oral: <2%), agitation (oral, rectal: <2%), amnesia (oral: <2%), apathy (oral: <2%), ataxia (oral: <2%), chills (oral: <2%), decreased cough reflex (<2%), euphoria (<2%), hallucination (oral: <2%), hypoesthesia (oral: <2%), lack of concentration (oral: <2%), lethargy (oral: <2%), malaise (oral: <2%), myoclonus (<2%), slurred speech (oral: <2%), vertigo (oral: <2%), voice disorder (oral: <2%), withdrawal syndrome (oral: <2%)
Dermatologic: Skin rash (oral, rectal: 3% to 10%), diaphoresis (oral, rectal: 2% to 10%), decubitus ulcer (oral: <2%), pallor (oral: <2%), pruritus (<2%)
Endocrine & metabolic: Amenorrhea (oral: <2%), decreased libido (oral, rectal: <2%), gynecomastia (oral: <2%), hyponatremia (oral: <2%), SIADH (oral: <2%)
Gastrointestinal: Abdominal pain (oral: 3% to 10%), anorexia (oral, rectal: 3% to 10%), diarrhea (3% to 10%), xerostomia (oral, rectal: 3% to 10%), biliary colic (oral: <2%), delayed gastric emptying (oral: <2%), dyspepsia (oral: <2%), dysphagia (oral: <2%), gastric atony (oral: <2%), gastroesophageal reflux disease (oral: <2%), hiccups (oral: <2%)
Genitourinary: Impotence (oral: <2%), urinary hesitancy (oral, rectal: <2%), urine abnormality (oral: <2%)
Hematologic & oncologic: Anemia (oral: 2% to <5%), thrombocytopenia (oral: <5%), leukopenia (oral: 2%)
Neuromuscular & skeletal: Back pain (oral: <2% to 10%), tremor (oral: 2%), asthenia (oral, rectal: ≤2%), arthralgia (oral: <2%), foot-drop (oral: <2%), ostealgia (oral: <2%)
Ophthalmic: Amblyopia (oral: <2%), blurred vision (oral: <2%), conjunctivitis (oral: <2%), diplopia (oral: <2%), miosis (oral, IV: <2%), nystagmus disorder (oral: <2%)
Respiratory: Dyspnea (3% to 10%), flu-like symptoms (oral: <2% to 10%), hypoventilation (<5%), asthma (oral: <2%), atelectasis (oral: <2%), hypoxia (oral: <2%), pulmonary edema (oral: <2%; includes noncardiogenic), respiratory depression (IV, epidural, intrathecal: <2%), respiratory insufficiency (oral: <2%), rhinitis (oral: <2%)
Miscellaneous: Accidental injury (oral: 2% to 10%), fever (oral: 2% to 10%), increased severity of condition (oral: 3%)
Frequency not defined:
Cardiovascular: Circulatory depression (oral, IV), orthostatic hypotension (IM, IV), peripheral vascular insufficiency (IV), phlebitis (IV), presyncope (oral, rectal), shock
Central nervous system: Abnormal gait (oral), altered mental status (IV), coma (oral), delirium (oral), disorientation (oral, rectal), disruption of body temperature regulation (IV, epidural, intrathecal), dysphoria, dyssynergia (oral), fear (IV), feeling abnormal (oral), increased catecholamines (IV, epidural, intrathecal), increased intracranial pressure (oral), mood changes (oral), nervousness (oral), paradoxical central nervous system stimulation (IV, epidural, intrathecal), sedated state (oral, rectal), toxic psychosis (IM, IV, epidural, intrathecal)
Dermatologic: Hemorrhagic urticaria (IV, rectal), urticaria, xeroderma (oral)
Endocrine & metabolic: Antidiuretic effect (oral, rectal), increased thirst (oral), weight loss (oral)
Gastrointestinal: Biliary tract spasm (rectal), decreased appetite (IV), dysgeusia (oral), gastroenteritis (oral), gastrointestinal hypermotility (IV; in patients with chronic ulcerative colitis), rectal disease (oral), toxic megacolon (IV; patients with chronic ulcerative colitis)
Genitourinary: Dysuria (oral), ejaculatory disorder (oral), erectile dysfunction (IV), hypogonadism (oral), oliguria, ureteral spasm (IV)
Hematologic & oncologic: Granuloma (IV, epidural, intrathecal)
Hepatic: Increased liver enzymes (oral)
Hypersensitivity: Nonimmune anaphylaxis (IM)
Local: Erythema at injection site (IV), induration at injection site (SC), local irritation (IV, epidural, intrathecal), local swelling (IV, intrathecal, epidural; genital swelling in males following infusion device implant surgery), pain at injection site (SC), residual mass at injection site (inflammatory; IV, epidural, intrathecal)
Neuromuscular & skeletal: Decreased bone mineral density (oral), laryngospasm (oral), muscle rigidity (oral), muscle spasm (IV, epidural, intrathecal; myoclonic spasm of lower extremities), muscle twitching (oral), vesicle sphincter spasm (IV)
Ophthalmic: Eye pain (oral), visual disturbance (oral, rectal)
Respiratory: Apnea (oral, IV)
Miscellaneous: Impaired physical performance (IV)
<1%, postmarketing, and/or case reports: Anaphylaxis, bronchospasm, dehydration, difficulty thinking, fatigue, hyperalgesia, hypersensitivity reaction, hypertonia, increased serum prolactin (Molitch 2008; Vuong 2010), intestinal obstruction, sepsis
Warnings/Precautions
Concerns related to adverse effects:
- CNS depression: May cause CNS depression, which may impair physical or mental abilities; patients must be cautioned about performing tasks which require mental alertness (eg, operating machinery or driving). Some dosage forms may be contraindicated in patients with severe CNS depression.
- Constipation: May cause constipation which may be problematic in patients with unstable angina and patients post-myocardial infarction. Consider preventive measures (eg, stool softener, increased fiber) to reduce the potential for constipation.
- Hypotension: May cause severe hypotension (including orthostatic hypotension and syncope); use with caution in patients with hypovolemia, cardiovascular disease (including acute MI), circulatory shock, or drugs that may exaggerate hypotensive effects (including phenothiazines or general anesthetics). Monitor for symptoms of hypotension following initiation or dose titration. Avoid use in patients with circulatory shock. Some dosage forms may be contraindicated in patients with cardiac arrhythmias or heart failure due to chronic lung disease.
- Phenanthrene hypersensitivity: Use with caution in patients with hypersensitivity reactions to other phenanthrene derivative opioid agonists (codeine, hydrocodone, hydromorphone, levorphanol, oxycodone, oxymorphone).
- Respiratory depression: [US Boxed Warning]: Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely for respiratory depression, especially during initiation or dose escalation. Swallow morphine ER formulations whole (or may sprinkle the contents of the capsule on applesauce and swallow without chewing); crushing, chewing, or dissolving the ER formulations can cause rapid release and absorption of a potentially fatal dose of morphine. Carbon dioxide retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
Disease-related concerns:
- Abdominal conditions: May obscure diagnosis or clinical course of patients with acute abdominal conditions.
- Adrenocortical insufficiency: Use with caution in patients with adrenal insufficiency, including Addison disease. Long-term opioid use may cause secondary hypogonadism, which may lead to sexual dysfunction, infertility, mood disorders, and osteoporosis (Brennan 2013).
- Biliary tract impairment: Use with caution in patients with biliary tract dysfunction or acute pancreatitis; opioids may cause constriction of sphincter of Oddi.
- CNS depression/coma: Avoid use in patients with impaired consciousness or coma, as these patients are susceptible to intracranial effects of CO2 retention.
- Delirium tremens: Use with caution in patients with delirium tremens. Some dosage forms may be contraindicated in patients with delirium tremens.
- Head trauma: Use with extreme caution in patients with head injury, intracranial lesions, or elevated intracranial pressure (ICP); exaggerated elevation of ICP may occur. Some dosage forms may be contraindicated in patients with increased intracranial or cerebrospinal pressure, head injuries, or brain tumor.
- Hepatic impairment: Use with caution in patients with severe hepatic impairment.
- Mental health conditions: Use opioids with caution for chronic pain in patients with mental health conditions (eg, depression, anxiety disorders, post-traumatic stress disorder) due to increased risk for opioid use disorder and overdose; more frequent monitoring is recommended (CDC [Dowell 2016]).
- Obesity: Use with caution in patients who are morbidly obese.
- Prostatic hyperplasia/urinary stricture: Use with caution in patients with prostatic hyperplasia and/or urinary stricture.
- Psychosis: Use with caution in patients with toxic psychosis.
- Renal impairment: Use with caution in patients with renal impairment.
- Respiratory disease: Use with caution and monitor for respiratory depression in patients with significant chronic obstructive pulmonary disease or cor pulmonale and patients having a substantially decreased respiratory reserve, hypoxia, hypercarbia, or preexisting respiratory depression, particularly when initiating therapy and titrating therapy; critical respiratory depression may occur, even at therapeutic dosages. Consider the use of alternative nonopioid analgesics in these patients.
- Sleep-related disorders: Opioid use increases the risk for sleep-related disorders (eg, central sleep apnea [CSA], hypoxemia) in a dose-dependent fashion. Use with caution for chronic pain and titrate dosage cautiously in patients with risk factors for sleep-disordered breathing (eg, heart failure, obesity). Consider dose reduction in patients presenting with CSA. Avoid opioids in patients with moderate to severe sleep-disordered breathing (CDC [Dowell 2016]).
- Seizure disorders: Use with caution in patients with seizure disorders; may cause or exacerbate preexisting seizures. Some dosage forms may be contraindicated in patients with seizure disorder.
- Thyroid dysfunction: Use with caution in patients with thyroid dysfunction.
Concurrent drug therapy issues:
- Benzodiazepines or other CNS depressants: [US Boxed Warning]: Concomitant use of opioids with benzodiazepines or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of morphine and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate. Limit dosage and durations to the minimum required and follow patients for signs and symptoms of respiratory depression and sedation. Some dosage forms may be contraindicated in patients with acute alcoholism.
- Ethanol use: Extended-release capsules: [US Boxed Warning]: Patients should not consume alcoholic beverages or medication containing ethanol while taking ER capsules; ethanol may increase morphine plasma levels, resulting in a potentially fatal overdose.
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Cachectic or debilitated patients: Use with caution in cachectic or debilitated patients; there is a greater potential for critical respiratory depression, even at therapeutic dosages. Consider the use of alternative nonopioid analgesics in these patients.
- Elderly: Use with caution in elderly patients; may be more sensitive to adverse effects, including life-threatening respiratory depression. Decrease initial dose. In the setting of chronic pain, monitor closely due to an increased potential for risks, including certain risks such as falls/fracture, cognitive impairment, and constipation. Clearance may also be reduced in older adults (with or without renal impairment) resulting in a narrow therapeutic window and increasing the risk for respiratory depression or overdose (CDC [Dowell 2016]). Consider the use of alternative nonopioid analgesics in these patients.
- Neonates: Neonatal withdrawal syndrome: [US Boxed Warning]: Prolonged maternal use of opioids during pregnancy can cause neonatal withdrawal syndrome in the newborn, which may be life-threatening if not recognized and treated according to protocols developed by neonatology experts. If prolonged opioid therapy is required in a pregnant woman, ensure treatment is available and warn patient of risk to the neonate. Signs and symptoms include irritability, hyperactivity, abnormal sleep pattern, high-pitched cry, tremor, vomiting, diarrhea, and failure to gain weight. Onset, duration, and severity depend on the drug used, duration of use, maternal dose, and rate of drug elimination by the newborn.
- Pediatric: Infants <3 months of age, especially if premature, are more susceptible to respiratory depression and/or apnea; use with caution and generally in reduced doses in this age group (APS 2008).
Dosage form specific issues:
- Infumorph, Duramorph, Mitigo: Neuroaxial administration: [US Boxed Warning]: Because of the risk of severe adverse effects when the epidural or intrathecal route of administration is employed, patients must be observed in a fully equipped and staffed environment for at least 24 hours after the initial dose. Single-dose Duramorph neuraxial administration may result in acute or delayed respiratory depression for up to 24 hours. Monitor patients receiving Infumorph or Mitigo for the first several days after catheter implantation. Naloxone injection should be immediately available. Thoracic epidural administration has been shown to dramatically increase the risk of early and late respiratory depression. High doses (> 20 mg/day) of neuraxial morphine may produce myoclonic events. Patients with reduced circulating blood volume or impaired myocardial function, or on concomitant sympatholytic drugs should be monitored for orthostatic hypotension, a frequent complication in single-dose neuraxial morphine.
- Benzyl alcohol and derivatives: Some dosage forms may contain sodium benzoate/benzoic acid; benzoic acid (benzoate) is a metabolite of benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC, 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol derivative with caution in neonates. See manufacturer's labeling.
- Extended-release formulations: Therapy should only be prescribed by health care professionals familiar with the use of potent opioids for chronic pain. Extended-release products are not interchangeable. When determining a generic equivalent or switching from one extended-release product to another, a thorough understanding of the pharmacokinetic properties is important in determining the proper generic equivalent or proper dose of the other extended-release product (review of the manufacturer's label may be necessary).
- Arymo ER: Moistened tablets may become sticky, leading to difficulty in swallowing the tablets; choking, gagging, regurgitation, and tablets getting stuck in the throat may occur. Tablet stickiness and swelling may also predispose patients to intestinal obstruction and exacerbation of diverticulitis. Do not to pre-soak, lick, or otherwise wet tablets prior to placing in the mouth; take one tablet at a time with enough water to ensure complete swallowing. Consider use of an alternative analgesic in patients who have difficulty swallowing and patients at risk for underlying GI disorders resulting in a small GI lumen (eg, esophageal cancer, colon cancer).
- Oral solution: Risk of medication errors: [US Boxed Warning]: Ensure accuracy when prescribing, dispensing, and administering morphine oral solution. Dosing errors due to confusion between mg and mL, and other morphine solutions of different concentrations, can result in accidental overdose and death. The 100 mg per 5 mL (20 mg/mL) is for use in opioid-tolerant patients only.
- Injections: [US Boxed Warning]: Because of delay in maximum CNS effect with IV administration (30 minutes), rapid IV administration may result in overdosing. Observe patients in a fully equipped and staffed environment for at least 24 hours after each test dose of Infumorph or Mitigo and, as indicated, for the first several days after surgery. Products are designed for administration by specific routes (ie, IV, intrathecal, epidural). Use caution when prescribing, dispensing, or administering to use formulations only by intended route(s). Rapid IV administration may result in chest wall rigidity. Use with caution when injecting IM into chilled areas or in patients with hypotension or shock (impaired perfusion may prevent complete absorption); if repeated injections are administered, an excessive amount may be suddenly absorbed if normal circulation is re-established.
- Infumorph, Mitigo: Should only be used in microinfusion devices; not for IV, IM, or SubQ administration or for single-dose administration. Administer intrathecal doses of 10 and 25 mg/mL to the lumbar area. Monitor closely, especially in the first 24 hours. Inflammatory masses (eg, granulomas), some resulting in severe neurologic impairment, have occurred when receiving Infumorph or Mitigo via indwelling intrathecal catheter; monitor carefully for new neurologic signs/symptoms.
- Product interchange: Improper or erroneous substitution of Infumorph or Mitigo for regular Duramorph is likely to result in serious overdosage, leading to seizures, respiratory depression, and possibly a fatal outcome.
- Sulfites: Some dosage forms may contain sulfites that may cause allergic reactions in sulfite sensitive patients.
Other warnings/precautions:
- Abrupt discontinuation/withdrawal: Abrupt discontinuation in patients who are physically dependent on opioids has been associated with serious withdrawal symptoms, uncontrolled pain, attempts to find other opioids (including illicit), and suicide. Use a collaborative, patient-specific taper schedule that minimizes the risk of withdrawal, considering factors such as current opioid dose, duration of use, type of pain, and physical and psychological factors. Monitor pain control, withdrawal symptoms, mood changes, suicidal ideation, and for use of other substances; provide care as needed. Concurrent use of mixed agonist/antagonist analgesics (eg, pentazocine, nalbuphine, butorphanol) or partial agonist (eg, buprenorphine) analgesics may also precipitate withdrawal symptoms and/or reduced analgesic efficacy in patients following prolonged therapy with mu opioid agonists.
- Abuse/misuse/diversion: [US Boxed Warning]: Morphine exposes patients and other users to the risks of addiction, abuse, and misuse, potentially leading to overdose and death. Assess each patient's risk prior to prescribing; monitor all patients regularly for development of these behaviors or conditions. Use with caution in patients with a history of drug abuse or acute alcoholism; potential for drug dependency exists. Other factors associated with increased risk include younger age, concomitant depression (major), and psychotropic medication use. Consider offering naloxone prescriptions in patients with factors associated with an increased risk for overdose, such as history of overdose or substance use disorder, higher opioid dosages (≥50 morphine milligram equivalents/day orally), and concomitant benzodiazepine use (CDC [Dowell 2016]).
- Accidental exposure: [US Boxed Warning]: Accidental ingestion of even one dose, especially in children, can result in a fatal overdose of morphine.
- Appropriate use: Chronic pain (outside of end-of-life or palliative care, active cancer treatment, sickle cell disease, or medication-assisted treatment for opioid use disorder) in outpatient setting in adults: Opioids should not be used as first-line therapy for chronic pain management (pain >3-month duration or beyond time of normal tissue healing) due to limited short-term benefits, undetermined long-term benefits, and association with serious risks (eg, overdose, MI, auto accidents, risk of developing opioid use disorder). Preferred management includes nonpharmacologic therapy and nonopioid therapy (eg, NSAIDS, acetaminophen, certain anticonvulsants and antidepressants). If opioid therapy is initiated, it should be combined with nonpharmacologic and nonopioid therapy, as appropriate. Prior to initiation, known risks of opioid therapy should be discussed and realistic treatment goals for pain/function should be established, including consideration for discontinuation if benefits do not outweigh risks. Therapy should be continued only if clinically meaningful improvement in pain/function outweighs risks. Therapy should be initiated at the lowest effective dosage using immediate-release opioids (instead of extended-release/long-acting opioids). Risk associated with use increases with higher opioid dosages. Risks and benefits should be re-evaluated when increasing dosage to ≥50 morphine milligram equivalents (MME)/day orally; dosages ≥90 MME/day orally should be avoided unless carefully justified (CDC [Dowell 2016]).
- Optimal regimen: An opioid-containing analgesic regimen should be tailored to each patient's needs and based upon the type of pain being treated (acute versus chronic), the route of administration, degree of tolerance for opioids (naive versus chronic user), age, weight, and medical condition. The optimal analgesic dose varies widely among patients; doses should be titrated to pain relief/prevention.
- Risk Evaluation and Mitigation Strategy (REMS): [US Boxed Warning]: To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the FDA has required a REMS for these products. Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS compliant education programs available to health care providers. Health care providers are strongly encouraged to complete a REMS-compliant education program; counsel patients and/or their caregivers, with every prescription, on safe use, serious risks, storage, and disposal of these products; emphasize to patients and their caregivers the importance of reading the Medication Guide every time it is provided by their pharmacist; and consider other tools to improve patient, household, and community safety.
- Surgery: Opioids decrease bowel motility; monitor for decreased bowel motility in postop patients receiving opioids. Use with caution in the perioperative setting; individualize treatment when transitioning from parenteral to oral analgesics. Some dosage forms may be contraindicated after biliary tract surgery, suspected surgical abdomen, or surgical anastomosis.
Monitoring Parameters
Pain control, respiratory and mental status; blood pressure; signs of misuse, abuse, and addiction; signs or symptoms of hypogonadism or hypoadrenalism (Brennan 2013)
Alternate recommendations: Chronic pain (long-term therapy outside of end-of-life or palliative care, active cancer treatment, sickle cell disease, or medication-assisted treatment for opioid use disorder): Evaluate benefits/risks of opioid therapy within 1 to 4 weeks of treatment initiation and with dose increases. Re-evaluate benefits/risks every 3 months during therapy or more frequently in patients at increased risk of overdose or opioid use disorder. Urine drug testing is recommended prior to initiation and with consideration for re-checking at least yearly (includes controlled prescription medications and illicit drugs of abuse). State prescription drug monitoring program (PDMP) data should be reviewed by clinicians prior to initiation and periodically during therapy (frequency ranging from every prescription to every 3 months) (Dowell [CDC 2016]).
Critically ill: The Numeric Rating Scale should be used in patients who are able to self-report pain. In patients who are unable to self-report pain, the Behavioral Pain Scale and the Critical-Care Pain Observational Tool can be used in intubated or nonintubated patients (SCCM [Devlin 2018]).
Epidural/intrathecal: Patients should be observed in a fully equipped and staffed environment for at least 24 hours following initiation, and as appropriate for the first several days after catheter implantation. Naloxone injection should be immediately available. Patient should remain in this environment for at least 24 hours following the initial dose. For patients receiving Infumorph or Mitigo via microinfusion device, patient may be observed, as appropriate, for the first several days after catheter implantation.
Alternative monitoring recommendations (Bujedo 2012): Epidural: Note: All patients receiving neuraxial opioids should be monitored for adequate ventilation (eg, respiratory rate, depth of respiration [without disturbing patient]), oxygenation (eg, pulse oximetry when appropriate), and level of consciousness.
Single dose: Monitor patient for a minimum of 24 hours after administration with a frequency of at least once per hour for the first 12 hours after administration, followed by at least once every 2 hours for the next 12 hours (ie, from 12 to 24 hours after administration). After 24 hours, frequency dictated by overall clinical condition and concurrent medications.
Continuous infusion or patient controlled epidural analgesia (PCEA): Monitor patient during the entire duration of the infusion with a frequency of at least once per hour for the first 12 hours, followed by at least once every 2 hours for the next 12 hours (ie, from 12 to 24 hours after administration). After 24 hours, monitor at least once every 4 hours. After discontinuation of infusion or PCEA, frequency dictated by overall clinical condition and concurrent medications.
Note: Also refer to institution specific protocols as appropriate.
Pregnancy
Pregnancy Considerations
Morphine crosses the placenta.
According to some studies, maternal use of opioids may be associated with birth defects (including neural tube defects, congenital heart defects, and gastroschisis), poor fetal growth, stillbirth, and preterm delivery (CDC [Dowell 2016]). Opioids used as part of obstetric analgesia/anesthesia during labor and delivery may temporarily affect the fetal heart rate (ACOG 209 2019).
[US Boxed Warning]: Prolonged use of morphine during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. If chronic opioid exposure occurs in pregnancy, adverse events in the newborn (including withdrawal) may occur (Chou 2009). Symptoms of neonatal abstinence syndrome (NAS) following opioid exposure may be autonomic (eg, fever, temperature instability), gastrointestinal (eg, diarrhea, vomiting, poor feeding/weight gain), or neurologic (eg, high-pitched crying, hyperactivity, increased muscle tone, increased wakefulness/abnormal sleep pattern, irritability, sneezing, seizure, tremor, yawning) (Dow 2012; Hudak 2012). Mothers who are physically dependent on opioids may give birth to infants who are also physically dependent. Opioids may cause respiratory depression and psycho-physiologic effects in the neonate; newborns of mothers receiving opioids during labor should be monitored.
Morphine injection is commonly used for the treatment of pain during labor and immediately postpartum (ACOG 209 2019). Not all dosage forms are appropriate for this use. Agents other than morphine are used to treat chronic non-cancer pain in pregnant women or those who may become pregnant (CDC [Dowell 2016]; Chou 2009).
Long-term opioid use may cause secondary hypogonadism, which may lead to sexual dysfunction or infertility in men and women (Brennan 2013).
Patient Education
What is this drug used for?
- It is used to ease pain.
Frequently reported side effects of this drug
- Dry mouth
- Nausea
- Vomiting
- Headache
- Anxiety
- Sweating a lot
- Diarrhea
- Lack of appetite
- Tablet shell in stool
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Trouble breathing
- Slow breathing
- Shallow breathing
- Noisy breathing
- Severe dizziness
- Passing out
- Chest pain
- Fast heartbeat
- Confusion
- Seizures
- Severe abdominal pain
- Severe constipation
- Swelling of arms or legs
- Chills
- Sore throat
- Painful urination
- Depression
- Burning or numbness feeling
- Adrenal gland problems like severe nausea, vomiting, severe dizziness, passing out, muscle weakness, severe fatigue, mood changes, lack of appetite, or weight loss
- Serotonin syndrome like dizziness, severe headache, agitation, sensing things that seem real but are not, fast heartbeat, abnormal heartbeat, flushing, tremors, sweating a lot, change in balance, severe nausea, or severe diarrhea
- Trouble controlling body movements
- Trouble urinating
- Muscle spasm
- Sexual dysfunction (males)
- Decreased sex drive
- No menstrual periods
- Trouble getting pregnant
- Severe loss of strength and energy
- Mood changes
- Thoughts of suicide
- Severe fatigue
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.