Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet Extended Release 24 Hour, Oral:
Sular: 8.5 mg
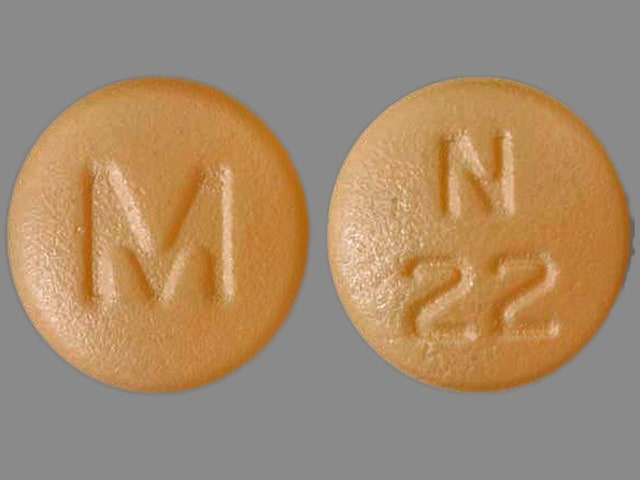
Sular: 17 mg [contains tartrazine (fd&c yellow #5)]
Sular: 34 mg
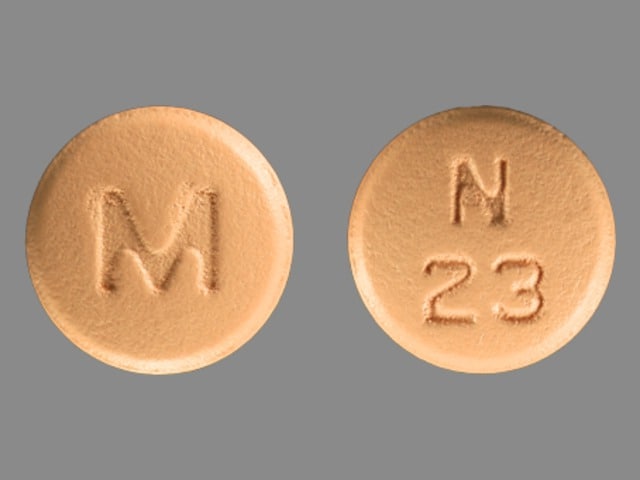
Generic: 8.5 mg, 17 mg, 20 mg, 25.5 mg, 30 mg, 34 mg, 40 mg
Pharmacology
Mechanism of Action
As a dihydropyridine calcium channel blocker, structurally similar to nifedipine, nisoldipine impedes the movement of calcium ions into vascular smooth muscle and cardiac muscle. Dihydropyridines are potent vasodilators and are not as likely to suppress cardiac contractility and slow cardiac conduction as other calcium antagonists such as verapamil and diltiazem; nisoldipine is 5-10 times as potent a vasodilator as nifedipine.
Pharmacokinetics/Pharmacodynamics
Absorption
Well absorbed. Peak concentrations significantly increased with high-lipid meals; however, AUC is reduced.
Metabolism
Extensively hepatic; 1 active metabolite (10% of activity of parent); first-pass effect
Excretion
Urine (60% to 80% as inactive metabolites); feces
Time to Peak
4-14 hours
Duration of Action
>24 hours
Half-Life Elimination
9-18 hours
Protein Binding
>99%
Use in Specific Populations
Special Populations: Hepatic Function Impairment
Liver cirrhosis: Increased plasma concentrations. Use lower starting and maintenance doses.
Special Populations: Elderly
Higher nisoldipine plasma concentrations (Cmax and AUC) have been found in elderly patients.
Use: Labeled Indications
Hypertension: Management of hypertension
Guideline recommendations: The 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults recommends if monotherapy is warranted, in the absence of comorbidities (eg, cerebrovascular disease, chronic kidney disease, diabetes, heart failure, ischemic heart disease, etc), that thiazide-like diuretics or dihydropyridine calcium channel blockers may be preferred options due to improved cardiovascular endpoints (eg, prevention of heart failure and stroke). ACE inhibitors and ARBs are also acceptable for monotherapy. Combination therapy may be required to achieve blood pressure goals and is initially preferred in patients at high risk (stage 2 hypertension or atherosclerotic cardiovascular disease [ASCVD] risk ≥10%) (ACC/AHA [Whelton 2017]).
Contraindications
Hypersensitivity to nisoldipine, any component of the formulation, or other dihydropyridine calcium channel blockers
Dosage and Administration
Dosing: Adult
Hypertension: Oral:
Sular (Geomatrix delivery system): Oral: Initial: 17 mg once daily, then increase by 8.5 mg/week (or longer intervals) to attain adequate control of blood pressure
Usual dose range: 17 to 34 mg once daily; doses >34 mg once daily are not recommended
Nisoldipine extended-release tablet (original formulation): Initial: 20 mg once daily; titrate weekly as needed based on patient response by 10 mg/week (or longer intervals); usual dosage range: 20 to 40 mg once daily; doses >60 mg once daily are not recommended
Conversion from nisoldipine extended-release (original formulation) to Sular Geomatrix delivery system:
|
Original Extended Release Formulation |
Sular Extended Release (Geomatrix delivery system) |
|---|---|
|
10 mg |
8.5 mg |
|
20 mg |
17 mg |
|
30 mg |
25.5 mg |
|
40 mg |
34 mg |
Table has been converted to the following text.
Nisoldipine Extended Release Dosing Equivalency
Original extended release formulation dose 10 mg equals
Sular Geomatrix dose 8.5 mg
Original extended release formulation dose 20 mg equals
Sular Geomatrix dose 17 mg
Original extended release formulation dose 30 mg equals
Sular Geomatrix dose 25.5 mg
Original extended release formulation dose 40 mg equals
Sular Geomatrix dose 34 mg
Dosing: Geriatric
Hypertension: Oral:
Sular (Geomatrix delivery system): Initial dose: 8.5 mg once daily; increase by 8.5 mg/week (or longer intervals) to attain adequate blood pressure control
Nisoldipine extended-release (original formulation): Initial dose: 10 mg once daily; increase by 10 mg/week (or longer intervals) to attain adequate blood pressure control.
Conversion from nisoldipine extended-release (original formulation) to Sular Geomatrix delivery system: Refer to adult dosing.
Administration
Oral: Administer at the same time each day to ensure minimal fluctuation of serum levels. Avoid high-fat diet. Administer on an empty stomach (1 hour before or 2 hours after a meal). Swallow whole; do not crush, break, split, or chew.
Dietary Considerations
Take on an empty stomach (1 hour before or 2 hours after a meal). Avoid grapefruit juice before and after dosing. Avoid grapefruit juice; avoid high-fat diet.
Storage
Store at 20°C to 25°C (68°F to 77°F). Protect from light; protect from moisture.
Nisoldipine Images
Drug Interactions
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Alpha1-Blockers: May enhance the hypotensive effect of Calcium Channel Blockers. Monitor therapy
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Aprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Atosiban: Calcium Channel Blockers may enhance the adverse/toxic effect of Atosiban. Specifically, there may be an increased risk for pulmonary edema and/or dyspnea. Monitor therapy
Barbiturates: May increase the metabolism of Calcium Channel Blockers. Management: Monitor for decreased therapeutic effects of calcium channel blockers with concomitant barbiturate therapy. Calcium channel blocker dose adjustments may be necessary. Nimodipine Canadian labeling contraindicates concomitant use with phenobarbital. Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Calcium Channel Blockers (Nondihydropyridine): Calcium Channel Blockers (Dihydropyridine) may enhance the hypotensive effect of Calcium Channel Blockers (Nondihydropyridine). Calcium Channel Blockers (Nondihydropyridine) may increase the serum concentration of Calcium Channel Blockers (Dihydropyridine). Monitor therapy
Calcium Salts: May diminish the therapeutic effect of Calcium Channel Blockers. Monitor therapy
Cimetidine: May increase the serum concentration of Calcium Channel Blockers. Management: Consider alternatives to cimetidine. If no suitable alternative exists, monitor for increased effects of calcium channel blockers following cimetidine initiation/dose increase, and decreased effects following cimetidine discontinuation/dose decrease. Consider therapy modification
Clofazimine: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Clopidogrel: Calcium Channel Blockers may diminish the therapeutic effect of Clopidogrel. Monitor therapy
Conivaptan: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
CycloSPORINE (Systemic): Calcium Channel Blockers (Dihydropyridine) may increase the serum concentration of CycloSPORINE (Systemic). CycloSPORINE (Systemic) may increase the serum concentration of Calcium Channel Blockers (Dihydropyridine). Monitor therapy
CYP3A4 Inducers (Moderate): May decrease the serum concentration of Nisoldipine. Avoid combination
CYP3A4 Inducers (Strong): May decrease the serum concentration of Nisoldipine. Avoid combination
CYP3A4 Inhibitors (Moderate): May decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CYP3A4 Inhibitors (Strong): May increase the serum concentration of Nisoldipine. Avoid combination
Dapoxetine: May enhance the orthostatic hypotensive effect of Calcium Channel Blockers. Monitor therapy
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
Duvelisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Erdafitinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fluconazole: May increase the serum concentration of Calcium Channel Blockers. Monitor therapy
Fosaprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fosnetupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fusidic Acid (Systemic): May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Grapefruit Juice: May increase the serum concentration of Nisoldipine. Avoid combination
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Idelalisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Ivosidenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Larotrectinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Macrolide Antibiotics: May decrease the metabolism of Calcium Channel Blockers. Management: Consider using a noninteracting macrolide. Felodipine Canadian labeling specifically recommends avoiding its use in combination with clarithromycin. Exceptions: Azithromycin (Systemic); Fidaxomicin; Roxithromycin; Spiramycin. Consider therapy modification
Magnesium Salts: Calcium Channel Blockers may enhance the adverse/toxic effect of Magnesium Salts. Magnesium Salts may enhance the hypotensive effect of Calcium Channel Blockers. Monitor therapy
Melatonin: May diminish the antihypertensive effect of Calcium Channel Blockers (Dihydropyridine). Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Netupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Neuromuscular-Blocking Agents (Nondepolarizing): Calcium Channel Blockers may enhance the neuromuscular-blocking effect of Neuromuscular-Blocking Agents (Nondepolarizing). Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Palbociclib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Simeprevir: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Sincalide: Drugs that Affect Gallbladder Function may diminish the therapeutic effect of Sincalide. Management: Consider discontinuing drugs that may affect gallbladder motility prior to the use of sincalide to stimulate gallbladder contraction. Consider therapy modification
Stiripentol: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Use of stiripentol with CYP3A4 substrates that are considered to have a narrow therapeutic index should be avoided due to the increased risk for adverse effects and toxicity. Any CYP3A4 substrate used with stiripentol requires closer monitoring. Consider therapy modification
Tacrolimus (Systemic): Calcium Channel Blockers (Dihydropyridine) may increase the serum concentration of Tacrolimus (Systemic). Monitor therapy
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
May lead to false-negative aldosterone/renin ratio (ARR) (Funder 2016)
Adverse Reactions
>10%:
Cardiovascular: Peripheral edema (7% to 29%; dose-related)
Central nervous system: Headache (22%)
1% to 10%:
Cardiovascular: Vasodilation (4%), palpitations (3%), exacerbation of angina pectoris (2%), chest pain (2%)
Central nervous system: Dizziness (3% to 10%)
Dermatologic: Skin rash (2%)
Gastrointestinal: Nausea (2%)
Respiratory: Pharyngitis (5%), sinusitis (3%)
<1%, postmarketing, and/or case reports: Abnormal dreams, abnormal hepatic function tests, abnormal T waves on ECG (flattening, inversion, non-specific changes), alopecia, amblyopia, amnesia, anemia, anorexia, anxiety, arthralgia, arthritis, asthma, ataxia, atrial fibrillation, blepharitis, bruise, cardiac failure (decompensated), cellulitis, cerebral ischemia, cerebrovascular accident, colitis, conjunctivitis, decreased libido, depression, dermal ulcer, diabetes mellitus, diaphoresis, diarrhea, drowsiness, dysgeusia, dyspepsia, dysphagia, dyspnea, dysuria, ejection murmur (systolic), epistaxis, exfoliative dermatitis, facial edema, fever, first degree atrioventricular block, flu-like symptoms, gastritis, gastrointestinal hemorrhage, gingival hyperplasia, glaucoma, glossitis, gout, gynecomastia, hematuria, hepatomegaly, herpes simplex infection, herpes zoster, hypersensitivity reaction (eg, angioedema, chest tightness, hypotension, shortness of breath, skin rash, tachycardia), hypertension, hypertonia, hypoesthesia, hypokalemia, hypotension, increased appetite, increased blood urea nitrogen, increased creatine phosphokinase, increased nonprotein nitrogen, increased serum creatinine, insomnia, jugular vein distention, keratoconjunctivitis, leukopenia, maculopapular rash, malaise, melena, migraine, myalgia, myasthenia, myocardial infarction, myositis, nocturia, oral mucosa ulcer, orthostatic hypotension, paresthesia, petechia, pleural effusion, pruritus, pustular rash, rales, retinal detachment, skin discoloration, skin photosensitivity, supraventricular tachycardia, syncope, tenosynovitis, thyroiditis, tremor, urinary frequency, urticaria, vaginal hemorrhage, venous insufficiency, ventricular premature contractions, vertigo, vision loss (temporary, unilateral), vitreous opacity, weight changes (gain/loss), wheezing (end inspiratory wheeze), xerostomia
Warnings/Precautions
Concerns related to adverse effects:
- Angina/MI: Increased angina and/or MI has occurred with initiation or dosage titration of dihydropyridine calcium channel blockers. Reflex tachycardia may occur resulting in angina and/or MI in patients with obstructive coronary disease, especially in the absence of concurrent beta-blockade.
- Hypotension/syncope: Symptomatic hypotension with or without syncope can rarely occur; blood pressure must be lowered at a rate appropriate for the patient's clinical condition. Monitor closely during initial dosing and with dosage adjustment.
- Peripheral edema: The most common side effect is peripheral edema; occurs within 2 to 3 weeks of starting therapy.
Disease-related concerns:
- Aortic stenosis: Use with caution in patients with severe aortic stenosis.
- Heart failure (HF): The ACC/AHA heart failure guidelines recommend to avoid use in patients with heart failure due to lack of benefit and/or worse outcomes with calcium channel blockers in general (ACC/AHA [Yancy 2013]).
- Hepatic impairment: Use with caution in patients with severe hepatic impairment; lower starting dose required.
- Hypertrophic cardiomyopathy (HCM) with outflow tract obstruction: Use with caution in patients with HCM and outflow tract obstruction since reduction in afterload may worsen symptoms associated with this condition.
Dosage form specific issues:
- Tartrazine: Some dosage forms contain tartrazine, which may cause allergic reactions in certain individuals (eg, aspirin hypersensitivity).
Special populations:
- Elderly: Use with caution in patients >65 years of age; lower starting dose recommended.
Monitoring Parameters
Blood pressure, heart rate
Hypertension: The 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults (ACC/AHA [Whelton 2017]):
Confirmed hypertension and known CVD or 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥10%: Target blood pressure <130/80 mm Hg is recommended
Confirmed hypertension without markers of increased ASCVD risk: Target blood pressure <130/80 mm Hg may be reasonable
Diabetes and hypertension: The American Diabetes Association (ADA) guidelines (ADA 2019):
Patients 18 to 65 years of age, without ASCVD, and 10-year ASCVD risk <15%: Target blood pressure <140/90 mm Hg is recommended
Patients 18 to 65 years of age and known ASCVD or 10-year ASCVD risk >15%: Target blood pressure <130/80 mm Hg may be appropriate if it can be safely attained
Patients >65 years of age (healthy or complex/intermediate health): Target blood pressure <140/90 mm Hg is recommended
Patients >65 years of age (very complex/poor health): Target blood pressure <150/90 mm Hg is recommended
Pregnancy
Pregnancy Considerations
Chronic maternal hypertension may increase the risk of birth defects, low birth weight, preterm delivery, stillbirth, and neonatal death. Actual fetal/neonatal risks may be related to duration and severity of maternal hypertension. Untreated hypertension may also increase the risks of adverse maternal outcomes, including gestational diabetes, myocardial infarction, preeclampsia, stroke, and delivery complications (ACOG 203 2019).
Calcium channel blockers may be used to treat hypertension in pregnant women; however, agents other than nisoldipine are more commonly used (ACOG 203 2019; ESC [Regitz-Zagrosek 2018]). Females with preexisting hypertension may continue their medication during pregnancy unless contraindications exist (ESC [Regitz-Zagrosek 2018]).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience sore throat or headache. Have patient report immediately to prescriber severe dizziness, passing out, shortness of breath, excessive weight gain, swelling of arms or legs, or chest pain (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for healthcare professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience and judgment in diagnosing, treating and advising patients.