Boxed Warning
Suicidal thoughts and behaviors:
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients older than 24 years; there was a reduction in risk with antidepressant use in patients 65 years and older. In patients of all ages who are started on antidepressant therapy, monitor closely for worsening and emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the health care provider. Olanzapine/fluoxetine is not approved for use in children younger than 10 years.
Increased mortality in elderly patients with dementia-related psychosis:
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Olanzapine/fluoxetine is not approved for the treatment of patients with dementia-related psychosis.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Capsule, oral: 3/25: Olanzapine 3 mg and fluoxetine 25 mg; 6/25: Olanzapine 6 mg and fluoxetine 25 mg; 6/50: Olanzapine 6 mg and fluoxetine 50 mg; 12/25: Olanzapine 12 mg and fluoxetine 25 mg; 12/50: Olanzapine 12 mg and fluoxetine 50 mg
Symbyax 3/25: Olanzapine 3 mg and fluoxetine 25 mg
Symbyax 6/25: Olanzapine 6 mg and fluoxetine 25 mg
Symbyax 6/50: Olanzapine 6 mg and fluoxetine 50 mg
Symbyax 12/25: Olanzapine 12 mg and fluoxetine 25 mg [DSC]
Symbyax 12/50: Olanzapine 12 mg and fluoxetine 50 mg
Pharmacology
Mechanism of Action
Olanzapine is a second generation thienobenzodiazepine antipsychotic which displays potent antagonism of serotonin 5-HT2A and 5-HT2C, dopamine D1-4, histamine H1 and alpha1-adrenergic receptors. Olanzapine shows moderate antagonism of 5-HT3 and muscarinic M1-5 receptors, and weak binding to GABA-A, BZD, and beta-adrenergic receptors. Fluoxetine inhibits CNS neuron serotonin reuptake; minimal or no effect on reuptake of norepinephrine or dopamine; does not significantly bind to alpha-adrenergic, histamine, or cholinergic receptors. The enhanced antidepressant effect of the combination may be due to synergistic increases in serotonin, norepinephrine, and dopamine.
Use: Labeled Indications
Depression, acute (associated with bipolar I disorder): Treatment of acute depressive episodes associated with bipolar I disorder
Depression, treatment-resistant: Treatment of treatment-resistant depression (eg, unresponsive to 2 trials of different antidepressants in the current episode)
Contraindications
Use of MAO inhibitors intended to treat psychiatric disorders (concurrently, within 5 weeks of discontinuing olanzapine/fluoxetine, or within 2 weeks of discontinuing the MAO inhibitor); initiation of olanzapine/fluoxetine in a patient receiving linezolid or intravenous methylene blue; use with pimozide or thioridazine (Note: Thioridazine should not be initiated until 5 weeks after the discontinuation of fluoxetine.)
Dosage and Administration
Dosing: Adult
Lower doses (olanzapine 3 to 6 mg/fluoxetine 25 mg) should be used in patients predisposed to hypotension, with hepatic impairment, with combined factors for reduced metabolism (females, the elderly, nonsmokers), or enhanced sensitivity to olanzapine; dose adjustments should be made with caution in this patient population.
Depression associated with bipolar I disorder: Initial: Olanzapine 6 mg/fluoxetine 25 mg once daily in the evening. Adjust dose based on response and tolerability. Usual dose: Olanzapine 6 to 12 mg/fluoxetine 25 to 50 mg. Safety of daily doses of olanzapine >18 mg/fluoxetine >75 mg have not been evaluated.
Treatment-resistant depression: Initial: Olanzapine 6 mg/fluoxetine 25 mg once daily in the evening. Adjust dose based on response and tolerability. Usual dose: Olanzapine 6 to 18 mg/fluoxetine 25 to 50 mg. Safety of daily doses of olanzapine >18 mg/fluoxetine >75 mg have not been evaluated.
Note: When using individual components of fluoxetine with olanzapine rather than fixed dose combination product (Symbyax), approximate dosage correspondence is as follows:
Olanzapine 2.5 mg + fluoxetine 20 mg = Symbyax 3/25
Olanzapine 5 mg + fluoxetine 20 mg = Symbyax 6/25
Olanzapine 12.5 mg + fluoxetine 20 mg = Symbyax 12/25
Olanzapine 5 mg + fluoxetine 50 mg = Symbyax 6/50
Olanzapine 12.5 mg + fluoxetine 50 mg = Symbyax 12/50
Discontinuation of therapy: When discontinuing antidepressant treatment that has lasted for >3 weeks and acute antipsychotic treatment, gradually taper the dose (eg, over 2 to 4 weeks) to withdrawal symptoms and detect reemerging symptoms (APA 2010; APA [Lehman 2004]; WFSBP [Bauer 2015]). Reasons for a slower antidepressant titration (eg, over 4 weeks) include use of a drug with a half-life <24 hours (eg, paroxetine, venlafaxine), prior history of antidepressant withdrawal symptoms, or high doses of antidepressants (APA 2010; Hirsch 2019). If intolerable withdrawal symptoms occur, resume the previously prescribed dose and/or decrease dose at a more gradual rate (Shelton 2001). Select patients (eg, those with a history of discontinuation syndrome) on long-term treatment (>6 months) may benefit from tapering over months to years with close monitoring to allow for detection of prodromal symptoms of disease recurrence (APA [Lehman 2004]; WFSBP [Bauer 2015]). Evidence supporting ideal taper rates is limited (Shelton 2001; WFSBP [Bauer 2015]).
MAO inhibitor recommendations:
Switching to or from an MAO inhibitor intended to treat psychiatric disorders:
Allow 14 days to elapse between discontinuing an MAO inhibitor intended to treat psychiatric disorders and initiation of olanzapine/fluoxetine.
Allow 5 weeks to elapse between discontinuing olanzapine/fluoxetine and initiation of an MAO inhibitor intended to treat psychiatric disorders.
Dosing: Geriatric
Oral: Initial: Olanzapine 3 to 6 mg/fluoxetine 25 mg once daily in the evening; use caution adjusting dose (metabolism may be decreased).
Discontinuation of therapy: Refer to adult dosing.
MAO inhibitor recommendations: Refer to adult dosing.
Dosing: Pediatric
Lower doses (olanzapine 3 to 6 mg/fluoxetine 25 mg) should be used in patients predisposed to hypotension, with hepatic impairment, with combined factors for reduced metabolism (females, nonsmokers), or enhanced sensitivity to olanzapine; dose adjustments should be made with caution in this patient population.
Depression associated with bipolar I disorder: Initial: Children and Adolescents 10 to 17 years: Oral: Olanzapine 3 mg /fluoxetine 25 mg in the evening. Adjust dose based on response and tolerability. Usual dose: Olanzapine 6 to 12 mg/fluoxetine 25 to 50 mg; safety of fluoxetine doses >50 mg in combination with olanzapine doses >12 mg has not been studied in pediatrics.
Note: When using individual components of fluoxetine with olanzapine rather than fixed-dose combination product (Symbyax), approximate dosage correspondence is as follows:
Olanzapine 2.5 mg + fluoxetine 20 mg = Symbyax 3/25
Olanzapine 5 mg + fluoxetine 20 mg = Symbyax 6/25
Olanzapine 12.5 mg + fluoxetine 20 mg = Symbyax 12/25
Olanzapine 5 mg + fluoxetine 50 mg = Symbyax 6/50
Olanzapine 12.5 mg + fluoxetine 50 mg = Symbyax 12/50
Discontinuation of therapy: Refer to adult dosing.
MAO inhibitor recommendations: Refer to adult dosing.
Administration
Administer capsules once daily in the evening; may be taken without regard to meals.
Storage
Store at 25°C (77°F); excursions are permitted between 15°C and 30°C (59°F and 86°F). Protect from moisture.
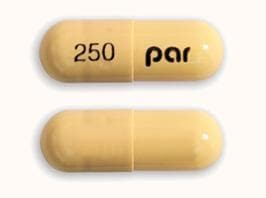
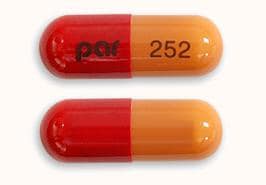
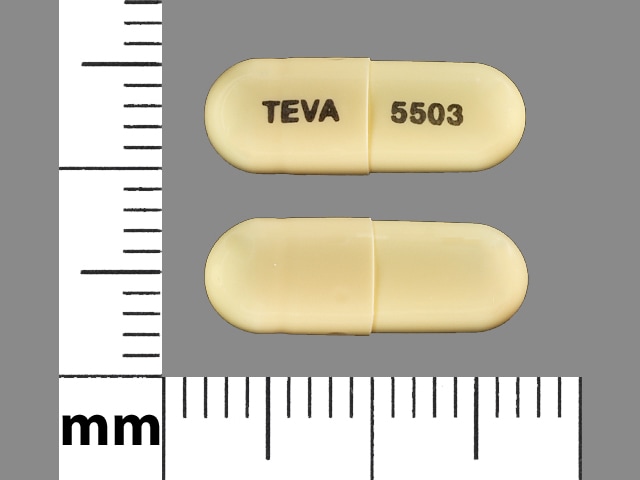
Olanzapine and Fluoxetine Images
Drug Interactions
Abiraterone Acetate: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Management: Avoid concurrent use of abiraterone with CYP2D6 substrates that have a narrow therapeutic index whenever possible. When concurrent use is not avoidable, monitor patients closely for signs/symptoms of toxicity. Consider therapy modification
Acalabrutinib: May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Acetylcholinesterase Inhibitors: May diminish the therapeutic effect of Anticholinergic Agents. Anticholinergic Agents may diminish the therapeutic effect of Acetylcholinesterase Inhibitors. Monitor therapy
Acetylcholinesterase Inhibitors (Central): May enhance the neurotoxic (central) effect of Antipsychotic Agents. Severe extrapyramidal symptoms have occurred in some patients. Monitor therapy
Aclidinium: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Agents with Antiplatelet Properties (e.g., P2Y12 inhibitors, NSAIDs, SSRIs, etc.): May enhance the antiplatelet effect of other Agents with Antiplatelet Properties. Monitor therapy
Alcohol (Ethyl): May enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Specifically, the risk of psychomotor impairment may be enhanced. Management: Patients receiving selective serotonin reuptake inhibitors should be advised to avoid alcohol. Monitor for increased psychomotor impairment in patients who consume alcohol during treatment with selective serotonin reuptake inhibitors. Consider therapy modification
Alizapride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Almotriptan: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Alosetron: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Alpelisib: May decrease the serum concentration of CYP2C9 Substrates (High risk with Inducers). Monitor therapy
Amifampridine: Agents With Seizure Threshold Lowering Potential may enhance the neuroexcitatory and/or seizure-potentiating effect of Amifampridine. Monitor therapy
Amisulpride: Antipsychotic Agents may enhance the adverse/toxic effect of Amisulpride. Management: Drugs listed as exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Avoid combination
Amisulpride: May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Avoid combination
Amphetamines: Antipsychotic Agents may diminish the stimulatory effect of Amphetamines. Monitor therapy
Amphetamines: May enhance the serotonergic effect of Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors). This could result in serotonin syndrome. Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors) may increase the serum concentration of Amphetamines. Management: Monitor for increased amphetamine toxicities, including signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability) when these agents are combined. Monitor therapy
Anticholinergic Agents: May enhance the adverse/toxic effect of other Anticholinergic Agents. Monitor therapy
Anticoagulants: Agents with Antiplatelet Properties may enhance the anticoagulant effect of Anticoagulants. Exceptions: Bemiparin; Enoxaparin; Heparin. Monitor therapy
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antiemetics (5HT3 Antagonists): May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Exceptions: Alosetron; Ondansetron; Ramosetron. Monitor therapy
Antihepaciviral Combination Products: May decrease the serum concentration of OLANZapine. Monitor therapy
Anti-Parkinson Agents (Dopamine Agonist): Antipsychotic Agents (Second Generation [Atypical]) may diminish the therapeutic effect of Anti-Parkinson Agents (Dopamine Agonist). Management: Consider using an alternative antipsychotic agent when possible in patients with Parkinson disease. If an atypical antipsychotic is necessary, consider using clozapine or quetiapine, which may convey the lowest interaction risk. Consider therapy modification
Antipsychotic Agents: Serotonergic Agents (High Risk) may enhance the adverse/toxic effect of Antipsychotic Agents. Specifically, serotonergic agents may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotic Agents may enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Monitor therapy
Apixaban: Agents with Antiplatelet Properties may enhance the adverse/toxic effect of Apixaban. Specifically, the risk for bleeding may be increased. Management: Carefully consider risks and benefits of this combination and monitor closely. Monitor therapy
ARIPiprazole: FLUoxetine may enhance the adverse/toxic effect of ARIPiprazole. Specifically, the risk of neuroleptic malignant syndrome may be increased. ARIPiprazole may enhance the serotonergic effect of FLUoxetine. This could result in serotonin syndrome. FLUoxetine may increase the serum concentration of ARIPiprazole. Management: Aripiprazole dose should be reduced by at least half, except when used adjunctively for depression. Consult full interaction monograph or aripiprazole prescribing information for complete details. Consider therapy modification
ARIPiprazole Lauroxil: CYP2D6 Inhibitors (Strong) may increase serum concentrations of the active metabolite(s) of ARIPiprazole Lauroxil. Management: Please refer to the full interaction monograph for details concerning the recommended dose adjustments. Consider therapy modification
Aspirin: Selective Serotonin Reuptake Inhibitors may enhance the antiplatelet effect of Aspirin. Monitor therapy
Asunaprevir: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Consider therapy modification
AtoMOXetine: CYP2D6 Inhibitors (Strong) may increase the serum concentration of AtoMOXetine. Management: Initiate atomoxetine at a reduced dose (adult doses -- patients up to 70kg: 0.5mg/kg/day; patients 70kg or more: 40mg/day) in patients receiving a strong CYP2D6 inhibitor. Consider therapy modification
Azelastine (Nasal): CNS Depressants may enhance the CNS depressant effect of Azelastine (Nasal). Avoid combination
Bemiparin: Agents with Antiplatelet Properties may enhance the anticoagulant effect of Bemiparin. Management: Avoid concomitant use of bemiparin with antiplatelet agents. If concomitant use is unavoidable, monitor closely for signs and symptoms of bleeding. Consider therapy modification
Benzodiazepines: OLANZapine may enhance the adverse/toxic effect of Benzodiazepines. Management: Avoid concomitant use of parenteral benzodiazepines and IM olanzapine due to risks of additive adverse events (e.g., cardiorespiratory depression). Olanzapine prescribing information provides no specific recommendations regarding oral administration. Avoid combination
Beta-Blockers: Selective Serotonin Reuptake Inhibitors may increase the serum concentration of Beta-Blockers. Exceptions: Acebutolol; Atenolol; Betaxolol (Ophthalmic); Betaxolol (Systemic); Bisoprolol; Carteolol (Ophthalmic); Esmolol; Labetalol; Levobunolol; Metipranolol; Nadolol; Sotalol. Monitor therapy
Blonanserin: CNS Depressants may enhance the CNS depressant effect of Blonanserin. Consider therapy modification
Blood Glucose Lowering Agents: Selective Serotonin Reuptake Inhibitors may enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Monitor therapy
Blood Pressure Lowering Agents: May enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Botulinum Toxin-Containing Products: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Brexanolone: CNS Depressants may enhance the CNS depressant effect of Brexanolone. Monitor therapy
Brexanolone: Selective Serotonin Reuptake Inhibitors may enhance the CNS depressant effect of Brexanolone. Monitor therapy
Brexpiprazole: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Brexpiprazole. Management: Reduce brexpiprazole dose to 50% of usual with a strong CYP2D6 inhibitor, reduce to 25% of usual if used with both a strong CYP2D6 inhibitor and a CYP3A4 inhibitor; these recommendations do not apply if treating major depressive disorder. Consider therapy modification
Brimonidine (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Broccoli: May decrease the serum concentration of CYP1A2 Substrates (High risk with Inducers). Monitor therapy
Bromopride: May enhance the adverse/toxic effect of Antipsychotic Agents. Avoid combination
Bromopride: May enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Avoid combination
Bromperidol: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
Buprenorphine: CNS Depressants may enhance the CNS depressant effect of Buprenorphine. Management: Consider reduced doses of other CNS depressants, and avoiding such drugs in patients at high risk of buprenorphine overuse/self-injection. Initiate buprenorphine at lower doses in patients already receiving CNS depressants. Consider therapy modification
BuPROPion: FLUoxetine may enhance the neuroexcitatory and/or seizure-potentiating effect of BuPROPion. BuPROPion may increase the serum concentration of FLUoxetine. Monitor therapy
BusPIRone: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Cannabidiol: CYP2C19 Inhibitors (Moderate) may increase the serum concentration of Cannabidiol. Monitor therapy
Cannabis: May decrease the serum concentration of CYP1A2 Substrates (High risk with Inducers). Monitor therapy
Cannabis: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
CarBAMazepine: FLUoxetine may increase the serum concentration of CarBAMazepine. Monitor therapy
CarBAMazepine: May decrease the serum concentration of OLANZapine. Monitor therapy
Cephalothin: Agents with Antiplatelet Properties may enhance the adverse/toxic effect of Cephalothin. Specifically, the risk for bleeding may be increased. Monitor therapy
Ceritinib: May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Chloral Betaine: May enhance the adverse/toxic effect of Anticholinergic Agents. Monitor therapy
Chlormethiazole: May enhance the CNS depressant effect of CNS Depressants. Management: Monitor closely for evidence of excessive CNS depression. The chlormethiazole labeling states that an appropriately reduced dose should be used if such a combination must be used. Consider therapy modification
Chlorphenesin Carbamate: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
Cilostazol: CYP2C19 Inhibitors (Moderate) may increase serum concentrations of the active metabolite(s) of Cilostazol. CYP2C19 Inhibitors (Moderate) may increase the serum concentration of Cilostazol. Management: Reduce the cilostazol dose to 50 mg twice daily in patients who are also receiving moderate inhibitors of CYP2C19. Monitor clinical response to cilostazol closely. Consider therapy modification
Cimetidine: May increase the serum concentration of FLUoxetine. Monitor therapy
Cimetropium: Anticholinergic Agents may enhance the anticholinergic effect of Cimetropium. Avoid combination
Citalopram: May enhance the antiplatelet effect of FLUoxetine. Citalopram may enhance the serotonergic effect of FLUoxetine. This could result in serotonin syndrome. FLUoxetine may increase the serum concentration of Citalopram. Management: Limit citalopram dose to a maximum of 20 mg/day. Monitor for signs and symptoms of bleeding, QTc interval prolongation, or serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor) if combined. Consider therapy modification
Clarithromycin: FLUoxetine may enhance the QTc-prolonging effect of Clarithromycin. Clarithromycin may increase the serum concentration of FLUoxetine. Monitor therapy
Clopidogrel: CYP2C19 Inhibitors (Moderate) may decrease serum concentrations of the active metabolite(s) of Clopidogrel. Management: Due to a risk for impaired clopidogrel effectiveness with such a combination, carefully consider the need for a moderate CYP2C19 inhibitor in patients receiving clopidogrel. Monitor patients closely for evidence of a diminished response to clopidogrel. Consider therapy modification
CloZAPine: CYP2D6 Inhibitors (Strong) may increase the serum concentration of CloZAPine. Monitor therapy
CloZAPine: QT-prolonging Antipsychotics (Moderate Risk) may enhance the QTc-prolonging effect of CloZAPine. Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
CNS Depressants: May enhance the adverse/toxic effect of other CNS Depressants. Monitor therapy
CNS Depressants: May enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Specifically, the risk of psychomotor impairment may be enhanced. Monitor therapy
Cobicistat: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Codeine: CYP2D6 Inhibitors (Strong) may diminish the therapeutic effect of Codeine. These CYP2D6 inhibitors may prevent the metabolic conversion of codeine to its active metabolite morphine. Consider therapy modification
Collagenase (Systemic): Agents with Antiplatelet Properties may enhance the adverse/toxic effect of Collagenase (Systemic). Specifically, the risk of injection site bruising and/or bleeding may be increased. Monitor therapy
Cyclobenzaprine: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
CYP1A2 Inducers (Moderate): May decrease the serum concentration of OLANZapine. Monitor therapy
CYP1A2 Inducers (Weak): May decrease the serum concentration of OLANZapine. Exceptions: CarBAMazepine; Ombitasvir, Paritaprevir, and Ritonavir; Ombitasvir, Paritaprevir, Ritonavir, and Dasabuvir; RifAMPin; Ritonavir; Tobacco (Smoked). Monitor therapy
CYP1A2 Inhibitors (Moderate): May increase the serum concentration of OLANZapine. Monitor therapy
CYP1A2 Inhibitors (Strong): May increase the serum concentration of OLANZapine. Monitor therapy
CYP2C19 Substrates (High risk with Inhibitors): CYP2C19 Inhibitors (Moderate) may decrease the metabolism of CYP2C19 Substrates (High risk with Inhibitors). Monitor therapy
CYP2C9 Inhibitors (Moderate): May decrease the metabolism of CYP2C9 Substrates (High risk with Inhibitors). Monitor therapy
CYP2D6 Inhibitors (Moderate): May decrease the metabolism of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
CYP2D6 Inhibitors (Strong): May decrease the metabolism of CYP2D6 Substrates (High risk with Inhibitors). Consider therapy modification
CYP2D6 Substrates (High risk with Inhibitors): CYP2D6 Inhibitors (Strong) may decrease the metabolism of CYP2D6 Substrates (High risk with Inhibitors). Exceptions: Ajmaline; Dapoxetine; Indoramin; Metoprolol; Tamoxifen; Timolol (Ophthalmic); Tropisetron. Consider therapy modification
Cyproheptadine: May diminish the therapeutic effect of Selective Serotonin Reuptake Inhibitors. Monitor therapy
Dabigatran Etexilate: Agents with Antiplatelet Properties may enhance the anticoagulant effect of Dabigatran Etexilate. Agents with Antiplatelet Properties may increase the serum concentration of Dabigatran Etexilate. This mechanism applies specifically to clopidogrel. Management: Carefully consider risks and benefits of this combination and monitor closely; Canadian labeling recommends avoiding prasugrel or ticagrelor. Monitor therapy
Dabrafenib: May decrease the serum concentration of CYP2C9 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP2C9 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Dacomitinib: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Management: Avoid concurrent use of dacomitinib with CYP2D6 subtrates that have a narrow therapeutic index. Consider therapy modification
Dapoxetine: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Do not use serotonergic agents (high risk) with dapoxetine or within 7 days of serotonergic agent discontinuation. Do not use dapoxetine within 14 days of monoamine oxidase inhibitor use. Dapoxetine labeling lists this combination as contraindicated. Avoid combination
Darunavir: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Dasatinib: May enhance the anticoagulant effect of Agents with Antiplatelet Properties. Management: Drugs listed as exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Monitor therapy
Deoxycholic Acid: Agents with Antiplatelet Properties may enhance the adverse/toxic effect of Deoxycholic Acid. Specifically, the risk for bleeding or bruising in the treatment area may be increased. Monitor therapy
Desmopressin: Selective Serotonin Reuptake Inhibitors may enhance the adverse/toxic effect of Desmopressin. Monitor therapy
Deutetrabenazine: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Deutetrabenazine. Management: The total daily dose of deutetrabenazine should not exceed 36 mg, and the maximum single dose of deutetrabenazine should not exceed 18 mg with concurrent use of a strong CYP2D6 inhibitor. Consider therapy modification
Dexmethylphenidate-Methylphenidate: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Dextromethorphan: May enhance the serotonergic effect of Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors). This could result in serotonin syndrome. Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors) may increase the serum concentration of Dextromethorphan. Management: Consider alternatives to this drug combination. If combined, monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes). Consider therapy modification
Dimethindene (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Domperidone: QT-prolonging Agents (Moderate Risk) may enhance the QTc-prolonging effect of Domperidone. Management: Consider alternatives to this drug combination. If combined, monitor for QTc interval prolongation and ventricular arrhythmias. Patients with additional risk factors for QTc prolongation may be at even higher risk. Consider therapy modification
DOXOrubicin (Conventional): CYP2D6 Inhibitors (Strong) may increase the serum concentration of DOXOrubicin (Conventional). Management: Seek alternatives to strong CYP2D6 inhibitors in patients treated with doxorubicin whenever possible. One U.S. manufacturer (Pfizer Inc.) recommends that these combinations be avoided. Consider therapy modification
Doxylamine: May enhance the CNS depressant effect of CNS Depressants. Management: The manufacturer of Diclegis (doxylamine/pyridoxine), intended for use in pregnancy, specifically states that use with other CNS depressants is not recommended. Monitor therapy
Dronabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Droperidol: QT-prolonging Antipsychotics (Moderate Risk) may enhance the QTc-prolonging effect of Droperidol. Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
DULoxetine: May enhance the antiplatelet effect of Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors). DULoxetine may enhance the serotonergic effect of Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors). This could result in serotonin syndrome. Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors) may increase the serum concentration of DULoxetine. Management: Monitor for increased duloxetine effects/toxicities and signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperthermia, tremor, mental status changes) when these agents are combined. In addition, monitor for signs and symptoms of bleeding. Monitor therapy
Edoxaban: Agents with Antiplatelet Properties may enhance the adverse/toxic effect of Edoxaban. Specifically, the risk of bleeding may be increased. Monitor therapy
Eletriptan: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Eliglustat: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Eliglustat. Management: Reduce the eliglustat dose to 84 mg daily. Avoid use of eliglustat in combination with a strong CYP2D6 inhibitor and a strong or moderate CYP3A4 inhibitor. Consider therapy modification
Eluxadoline: Anticholinergic Agents may enhance the constipating effect of Eluxadoline. Avoid combination
Enoxaparin: Agents with Antiplatelet Properties may enhance the anticoagulant effect of Enoxaparin. Management: Discontinue antiplatelet agents prior to initiating enoxaparin whenever possible. If concomitant administration is unavoidable, monitor closely for signs and symptoms of bleeding. Consider therapy modification
Enzalutamide: May decrease the serum concentration of CYP2C9 Substrates (High risk with Inducers). Management: Concurrent use of enzalutamide with CYP2C9 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP2C9 substrate should be performed with caution and close monitoring. Consider therapy modification
Ergot Derivatives: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Exceptions: Nicergoline. Monitor therapy
Esketamine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Fat Emulsion (Fish Oil Based): May enhance the adverse/toxic effect of Agents with Antiplatelet Properties. Monitor therapy
Fesoterodine: CYP2D6 Inhibitors may increase serum concentrations of the active metabolite(s) of Fesoterodine. Monitor therapy
Fexinidazole [INT]: May enhance the QTc-prolonging effect of QT-prolonging Agents (Moderate Risk). Avoid combination
Flibanserin: CYP2C19 Inhibitors (Moderate) may increase the serum concentration of Flibanserin. Monitor therapy
Flupentixol: QT-prolonging Antipsychotics (Moderate Risk) may enhance the QTc-prolonging effect of Flupentixol. Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Fosphenytoin: May enhance the QTc-prolonging effect of FLUoxetine. FLUoxetine may increase the serum concentration of Fosphenytoin. Consider therapy modification
Galantamine: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Galantamine. Monitor therapy
Gastrointestinal Agents (Prokinetic): Anticholinergic Agents may diminish the therapeutic effect of Gastrointestinal Agents (Prokinetic). Monitor therapy
Gilteritinib: May diminish the therapeutic effect of Selective Serotonin Reuptake Inhibitors. Management: Avoid use of this combination if possible. If the combination cannot be avoided, monitor closely for evidence of reduced response to the selective serotonin reuptake inhibitor. Consider therapy modification
Glucagon: Anticholinergic Agents may enhance the adverse/toxic effect of Glucagon. Specifically, the risk of gastrointestinal adverse effects may be increased. Monitor therapy
Glucosamine: May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Glycopyrrolate (Oral Inhalation): Anticholinergic Agents may enhance the anticholinergic effect of Glycopyrrolate (Oral Inhalation). Avoid combination
Glycopyrronium (Topical): May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Guanethidine: Antipsychotic Agents may diminish the therapeutic effect of Guanethidine. Monitor therapy
Haloperidol: FLUoxetine may enhance the QTc-prolonging effect of Haloperidol. FLUoxetine may increase the serum concentration of Haloperidol. Monitor therapy
Heparin: Agents with Antiplatelet Properties may enhance the anticoagulant effect of Heparin. Management: Decrease the dose of heparin or agents with antiplatelet properties if coadministration is required. Consider therapy modification
Herbs (Anticoagulant/Antiplatelet Properties) (eg, Alfalfa, Anise, Bilberry): May enhance the adverse/toxic effect of Agents with Antiplatelet Properties. Bleeding may occur. Management: Avoid combination when possible. If used, monitor more closely for evidence of bleeding. Discontinue herbal products with anticoagulant or antiplatelet actions 2 weeks prior to surgical, dental, or invasive procedures. Consider therapy modification
HYDROcodone: CNS Depressants may enhance the CNS depressant effect of HYDROcodone. Management: Avoid concomitant use of hydrocodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
HydrOXYzine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Ibritumomab Tiuxetan: Agents with Antiplatelet Properties may enhance the adverse/toxic effect of Ibritumomab Tiuxetan. Both agents may contribute to impaired platelet function and an increased risk of bleeding. Monitor therapy
Ibrutinib: May enhance the adverse/toxic effect of Agents with Antiplatelet Properties. Monitor therapy
Iloperidone: CYP2D6 Inhibitors (Strong) may increase serum concentrations of the active metabolite(s) of Iloperidone. Specifically, concentrations of the metabolite P88 may be increased. CYP2D6 Inhibitors (Strong) may decrease serum concentrations of the active metabolite(s) of Iloperidone. Specifically, concentrations of the metabolite P95 may be decreased. CYP2D6 Inhibitors (Strong) may increase the serum concentration of Iloperidone. Management: Reduce iloperidone dose by half when administered with a strong CYP2D6 inhibitor. Consider therapy modification
Imatinib: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Indoramin: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Indoramin. Monitor therapy
Inotersen: May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Ioflupane I 123: Selective Serotonin Reuptake Inhibitors may diminish the diagnostic effect of Ioflupane I 123. Monitor therapy
Iohexol: Agents With Seizure Threshold Lowering Potential may enhance the adverse/toxic effect of Iohexol. Specifically, the risk for seizures may be increased. Management: Discontinue agents that may lower the seizure threshold 48 hours prior to intrathecal use of iohexol. Wait at least 24 hours after the procedure to resume such agents. In nonelective procedures, consider use of prophylactic anticonvulsants. Consider therapy modification
Iomeprol: Agents With Seizure Threshold Lowering Potential may enhance the adverse/toxic effect of Iomeprol. Specifically, the risk for seizures may be increased. Management: Discontinue agents that may lower the seizure threshold 48 hours prior to intrathecal use of iomeprol. Wait at least 24 hours after the procedure to resume such agents. In nonelective procedures, consider use of prophylactic anticonvulsants. Consider therapy modification
Iopamidol: Agents With Seizure Threshold Lowering Potential may enhance the adverse/toxic effect of Iopamidol. Specifically, the risk for seizures may be increased. Management: Discontinue agents that may lower the seizure threshold 48 hours prior to intrathecal use of iopamidol. Wait at least 24 hours after the procedure to resume such agents. In nonelective procedures, consider use of prophylactic anticonvulsants. Consider therapy modification
Ipratropium (Oral Inhalation): May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Itopride: Anticholinergic Agents may diminish the therapeutic effect of Itopride. Monitor therapy
Kava Kava: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
LamoTRIgine: May enhance the sedative effect of OLANZapine. Monitor therapy
Lasmiditan: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Lemborexant: May enhance the CNS depressant effect of CNS Depressants. Management: Dosage adjustments of lemborexant and of concomitant CNS depressants may be necessary when administered together because of potentially additive CNS depressant effects. Close monitoring for CNS depressant effects is necessary. Consider therapy modification
Levosulpiride: Anticholinergic Agents may diminish the therapeutic effect of Levosulpiride. Avoid combination
Limaprost: May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Linezolid: May enhance the serotonergic effect of Selective Serotonin Reuptake Inhibitors. This could result in serotonin syndrome. Avoid combination
Lithium: May enhance the neurotoxic effect of Antipsychotic Agents. Lithium may decrease the serum concentration of Antipsychotic Agents. Specifically noted with chlorpromazine. Monitor therapy
Lofexidine: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Lofexidine. Monitor therapy
Lorcaserin: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Lumacaftor and Ivacaftor: May decrease the serum concentration of CYP2C9 Substrates (High Risk with Inhibitors or Inducers). Lumacaftor and Ivacaftor may increase the serum concentration of CYP2C9 Substrates (High Risk with Inhibitors or Inducers). Monitor therapy
Lumefantrine: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Magnesium Sulfate: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Mequitazine: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Mequitazine. Avoid combination
Metaxalone: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Methotrimeprazine: CNS Depressants may enhance the CNS depressant effect of Methotrimeprazine. Methotrimeprazine may enhance the CNS depressant effect of CNS Depressants. Management: Reduce adult dose of CNS depressant agents by 50% with initiation of concomitant methotrimeprazine therapy. Further CNS depressant dosage adjustments should be initiated only after clinically effective methotrimeprazine dose is established. Consider therapy modification
Methylene Blue: Selective Serotonin Reuptake Inhibitors may enhance the serotonergic effect of Methylene Blue. This could result in serotonin syndrome. Avoid combination
Methylphenidate: Antipsychotic Agents may enhance the adverse/toxic effect of Methylphenidate. Methylphenidate may enhance the adverse/toxic effect of Antipsychotic Agents. Monitor therapy
Metoclopramide: May enhance the adverse/toxic effect of Antipsychotic Agents. Avoid combination
Metoprolol: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Metoprolol. Monitor therapy
MetyroSINE: CNS Depressants may enhance the sedative effect of MetyroSINE. Monitor therapy
MetyroSINE: May enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Monitor therapy
MetyroSINE: May enhance the adverse/toxic effect of Antipsychotic Agents. Monitor therapy
Mianserin: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
MiFEPRIStone: May increase the serum concentration of CYP2C9 Substrates (High risk with Inhibitors). Management: Use CYP2C9 substrates at the lowest recommended dose, and monitor closely for adverse effects, during and in the 2 weeks following mifepristone treatment. Consider therapy modification
Minocycline (Systemic): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Mirabegron: Anticholinergic Agents may enhance the adverse/toxic effect of Mirabegron. Monitor therapy
Monoamine Oxidase Inhibitors (Antidepressant): Selective Serotonin Reuptake Inhibitors may enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
Multivitamins/Fluoride (with ADE): May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Multivitamins/Minerals (with ADEK, Folate, Iron): May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Multivitamins/Minerals (with AE, No Iron): May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Nabilone: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Nebivolol: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Nebivolol. Monitor therapy
Nefazodone: May enhance the serotonergic effect of Selective Serotonin Reuptake Inhibitors. This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Nicergoline: CYP2D6 Inhibitors (Strong) may increase serum concentrations of the active metabolite(s) of Nicergoline. Specifically, concentrations of the MMDL metabolite may be increased. CYP2D6 Inhibitors (Strong) may decrease serum concentrations of the active metabolite(s) of Nicergoline. Specifically, concentrations of the MDL metabolite may be decreased. Monitor therapy
NIFEdipine: FLUoxetine may enhance the adverse/toxic effect of NIFEdipine. Monitor therapy
NiMODipine: FLUoxetine may increase the serum concentration of NiMODipine. Monitor therapy
Nitroglycerin: Anticholinergic Agents may decrease the absorption of Nitroglycerin. Specifically, anticholinergic agents may decrease the dissolution of sublingual nitroglycerin tablets, possibly impairing or slowing nitroglycerin absorption. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents (COX-2 Selective): Selective Serotonin Reuptake Inhibitors may enhance the antiplatelet effect of Nonsteroidal Anti-Inflammatory Agents (COX-2 Selective). Nonsteroidal Anti-Inflammatory Agents (COX-2 Selective) may diminish the therapeutic effect of Selective Serotonin Reuptake Inhibitors. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents (Nonselective): Selective Serotonin Reuptake Inhibitors may enhance the antiplatelet effect of Nonsteroidal Anti-Inflammatory Agents (Nonselective). Nonsteroidal Anti-Inflammatory Agents (Nonselective) may diminish the therapeutic effect of Selective Serotonin Reuptake Inhibitors. Management: Consider alternatives to NSAIDs. Monitor for evidence of bleeding and diminished antidepressant effects. It is unclear whether COX-2-selective NSAIDs reduce risk. Exceptions: Diclofenac (Topical); Ibuprofen (Topical); Piroxicam (Topical). Consider therapy modification
Nonsteroidal Anti-Inflammatory Agents (Topical): May enhance the antiplatelet effect of Selective Serotonin Reuptake Inhibitors. Monitor therapy
Obinutuzumab: Agents with Antiplatelet Properties may enhance the adverse/toxic effect of Obinutuzumab. Specifically, the risk of serious bleeding-related events may be increased. Monitor therapy
Omega-3 Fatty Acids: May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Ondansetron: May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Ondansetron: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Opioid Agonists: CNS Depressants may enhance the CNS depressant effect of Opioid Agonists. Management: Avoid concomitant use of opioid agonists and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Orphenadrine: CNS Depressants may enhance the CNS depressant effect of Orphenadrine. Avoid combination
Oxatomide: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Oxitriptan: Serotonergic Agents (High Risk) may enhance the serotonergic effect of Oxitriptan. This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Oxomemazine: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
OxyCODONE: CNS Depressants may enhance the CNS depressant effect of OxyCODONE. Management: Avoid concomitant use of oxycodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Panobinostat: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Paraldehyde: CNS Depressants may enhance the CNS depressant effect of Paraldehyde. Avoid combination
PARoxetine: FLUoxetine may enhance the antiplatelet effect of PARoxetine. FLUoxetine may enhance the serotonergic effect of PARoxetine. This could result in serotonin syndrome. FLUoxetine may increase the serum concentration of PARoxetine. PARoxetine may increase the serum concentration of FLUoxetine. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, mental status changes), bleeding, and increased SSRI toxicities when these agents are combined. Monitor therapy
Peginterferon Alfa-2b: May decrease the serum concentration of FLUoxetine. Monitor therapy
Pentamidine (Systemic): May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Pentosan Polysulfate Sodium: May enhance the adverse/toxic effect of Agents with Antiplatelet Properties. Specifically, the risk of bleeding may be increased by concurrent use of these agents. Monitor therapy
Pentoxifylline: May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Perampanel: May enhance the CNS depressant effect of CNS Depressants. Management: Patients taking perampanel with any other drug that has CNS depressant activities should avoid complex and high-risk activities, particularly those such as driving that require alertness and coordination, until they have experience using the combination. Consider therapy modification
Perhexiline: CYP2D6 Inhibitors may increase the serum concentration of Perhexiline. Management: Consider alternatives to this combination if possible. If combined, monitor for increased perhexiline serum concentrations and toxicities (eg, hypoglycemia, neuropathy, liver dysfunction). Perhexiline dose reductions will likely be required. Consider therapy modification
Phenytoin: FLUoxetine may increase the serum concentration of Phenytoin. Monitor therapy
Pimozide: FLUoxetine may enhance the QTc-prolonging effect of Pimozide. FLUoxetine may increase the serum concentration of Pimozide. Avoid combination
Piribedil: Antipsychotic Agents may diminish the therapeutic effect of Piribedil. Piribedil may diminish the therapeutic effect of Antipsychotic Agents. Management: Use of piribedil with antiemetic neuroleptics is contraindicated, and use with antipsychotic neuroleptics, except for clozapine, is not recommended. Avoid combination
Pitolisant: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Pitolisant. Management: Reduce the pitolisant dose by 50% if a strong CYP2D6 inhibitor is initiated. For patients receiving strong CYP2D6 inhibitors, initiate pitolisant at 8.9 mg once daily and increase after 7 days to a maximum of 17.8 mg once daily. Consider therapy modification
Potassium Chloride: Anticholinergic Agents may enhance the ulcerogenic effect of Potassium Chloride. Management: Patients on drugs with substantial anticholinergic effects should avoid using any solid oral dosage form of potassium chloride. Avoid combination
Potassium Citrate: Anticholinergic Agents may enhance the ulcerogenic effect of Potassium Citrate. Avoid combination
Pramlintide: May enhance the anticholinergic effect of Anticholinergic Agents. These effects are specific to the GI tract. Consider therapy modification
Primaquine: CYP2D6 Inhibitors (Strong) may diminish the therapeutic effect of Primaquine. Management: Monitor for signs and symptoms of possible treatment failure with primaquine in patients who are taking strong CYP2D6 inhibitors. If efficacy of primaquine is compromised, may consider adjusting therapies. Consider therapy modification
Propafenone: May enhance the QTc-prolonging effect of FLUoxetine. FLUoxetine may increase the serum concentration of Propafenone. Monitor therapy
Prostacyclin Analogues: May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
QT-prolonging Agents (Highest Risk): May enhance the QTc-prolonging effect of OLANZapine. Management: Consider alternatives to this combination. Patients with other risk factors (eg, older age, female sex, bradycardia, hypokalemia, hypomagnesemia, heart disease, and higher drug concentrations) are likely at greater risk for these toxicities. Consider therapy modification
QT-prolonging Antidepressants (Moderate Risk): QT-prolonging Antipsychotics (Moderate Risk) may enhance the QTc-prolonging effect of QT-prolonging Antidepressants (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
QT-prolonging Antipsychotics (Moderate Risk): May enhance the QTc-prolonging effect of OLANZapine. Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Exceptions: Amisulpride; CloZAPine; Droperidol; Flupentixol; OLANZapine; Pimozide. Monitor therapy
QT-prolonging Class IC Antiarrhythmics (Moderate Risk): May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
QT-prolonging Kinase Inhibitors (Moderate Risk): QT-prolonging Antipsychotics (Moderate Risk) may enhance the QTc-prolonging effect of QT-prolonging Kinase Inhibitors (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
QT-prolonging Miscellaneous Agents (Moderate Risk): May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Exceptions: Domperidone. Monitor therapy
QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk): May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
QT-prolonging Quinolone Antibiotics (Moderate Risk): May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Quinagolide: Antipsychotic Agents may diminish the therapeutic effect of Quinagolide. Monitor therapy
Ramosetron: Anticholinergic Agents may enhance the constipating effect of Ramosetron. Monitor therapy
Ramosetron: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Rasagiline: Selective Serotonin Reuptake Inhibitors may enhance the serotonergic effect of Rasagiline. This could result in serotonin syndrome. Avoid combination
Revefenacin: Anticholinergic Agents may enhance the anticholinergic effect of Revefenacin. Avoid combination
RifAMPin: May decrease the serum concentration of OLANZapine. Monitor therapy
Rifapentine: May decrease the serum concentration of CYP2C9 Substrates (High risk with Inducers). Monitor therapy
Ritonavir: May decrease the serum concentration of OLANZapine. Monitor therapy
Rivaroxaban: Agents with Antiplatelet Properties may enhance the anticoagulant effect of Rivaroxaban. Management: Carefully consider risks and benefits of this combination and monitor closely; Canadian labeling recommends avoiding prasugrel or ticagrelor. Monitor therapy
Rufinamide: May enhance the adverse/toxic effect of CNS Depressants. Specifically, sleepiness and dizziness may be enhanced. Monitor therapy
Safinamide: May enhance the serotonergic effect of Selective Serotonin Reuptake Inhibitors. This could result in serotonin syndrome. Management: Use the lowest effective dose of SSRIs in patients treated with safinamide and monitor for signs and symptoms of serotonin syndrome/serotonin toxicity. Consider therapy modification
Salicylates: Agents with Antiplatelet Properties may enhance the adverse/toxic effect of Salicylates. Increased risk of bleeding may result. Monitor therapy
Saquinavir: QT-prolonging Antipsychotics (Moderate Risk) may enhance the QTc-prolonging effect of Saquinavir. Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Secretin: Anticholinergic Agents may diminish the therapeutic effect of Secretin. Management: Avoid concomitant use of anticholinergic agents and secretin. Discontinue anticholinergic agents at least 5 half-lives prior to administration of secretin. Consider therapy modification
Selective Serotonin Reuptake Inhibitors: CNS Depressants may enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Specifically, the risk of psychomotor impairment may be enhanced. Monitor therapy
Selective Serotonin Reuptake Inhibitors: May enhance the antiplatelet effect of other Selective Serotonin Reuptake Inhibitors. Selective Serotonin Reuptake Inhibitors may enhance the serotonergic effect of other Selective Serotonin Reuptake Inhibitors. This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, mental status changes) when these agents are combined. In addition, monitor for signs and symptoms of bleeding. Exceptions: Citalopram; Dapoxetine; Vortioxetine. Monitor therapy
Selegiline: Selective Serotonin Reuptake Inhibitors may enhance the serotonergic effect of Selegiline. This could result in serotonin syndrome. Avoid combination
Serotonergic Agents (High Risk): May enhance the adverse/toxic effect of Antipsychotic Agents. Specifically, serotonergic agents may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotic Agents may enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Monitor therapy
Serotonergic Agents (High Risk, Miscellaneous): May enhance the serotonergic effect of Selective Serotonin Reuptake Inhibitors. This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Serotonergic Non-Opioid CNS Depressants: Selective Serotonin Reuptake Inhibitors may enhance the serotonergic effect of Serotonergic Non-Opioid CNS Depressants. This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Serotonin 5-HT1D Receptor Agonists (Triptans): May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Exceptions: Almotriptan; Eletriptan. Monitor therapy
Serotonin/Norepinephrine Reuptake Inhibitors: Selective Serotonin Reuptake Inhibitors may enhance the antiplatelet effect of Serotonin/Norepinephrine Reuptake Inhibitors. Selective Serotonin Reuptake Inhibitors may enhance the serotonergic effect of Serotonin/Norepinephrine Reuptake Inhibitors. This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, mental status changes) when these agents are combined. In addition, monitor for signs and symptoms of bleeding. Exceptions: DULoxetine. Monitor therapy
Sodium Oxybate: May enhance the CNS depressant effect of CNS Depressants. Management: Consider alternatives to combined use. When combined use is needed, consider minimizing doses of one or more drugs. Use of sodium oxybate with alcohol or sedative hypnotics is contraindicated. Consider therapy modification
St John's Wort: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. St John's Wort may decrease the serum concentration of Serotonergic Agents (High Risk). Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Sulpiride: Antipsychotic Agents may enhance the adverse/toxic effect of Sulpiride. Avoid combination
Suvorexant: CNS Depressants may enhance the CNS depressant effect of Suvorexant. Management: Dose reduction of suvorexant and/or any other CNS depressant may be necessary. Use of suvorexant with alcohol is not recommended, and the use of suvorexant with any other drug to treat insomnia is not recommended. Consider therapy modification
Syrian Rue: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Tamoxifen: CYP2D6 Inhibitors (Strong) may decrease serum concentrations of the active metabolite(s) of Tamoxifen. Specifically, strong CYP2D6 inhibitors may decrease the metabolic formation of highly potent active metabolites. Avoid combination
Tamsulosin: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Tamsulosin. Monitor therapy
Tapentadol: May enhance the CNS depressant effect of CNS Depressants. Management: Avoid concomitant use of tapentadol and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Tetrabenazine: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Tetrabenazine. Specifically, concentrations of the active alpha- and beta-dihydrotetrabenazine metabolites may be increased. Management: Tetrabenazine adult dose should be reduced by 50% when starting a strong CYP2D6 inhibitor. Maximum tetrabenazine adult dose is 50 mg/day when used with a strong CYP2D6 inhibitor. Consider therapy modification
Tetrahydrocannabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Tetrahydrocannabinol and Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Thalidomide: CNS Depressants may enhance the CNS depressant effect of Thalidomide. Avoid combination
Thiazide and Thiazide-Like Diuretics: Selective Serotonin Reuptake Inhibitors may enhance the hyponatremic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Thiazide and Thiazide-Like Diuretics: Anticholinergic Agents may increase the serum concentration of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Thioridazine: FLUoxetine may enhance the QTc-prolonging effect of Thioridazine. FLUoxetine may increase the serum concentration of Thioridazine. Avoid combination
Thrombolytic Agents: Agents with Antiplatelet Properties may enhance the anticoagulant effect of Thrombolytic Agents. Monitor therapy
Thyroid Products: Selective Serotonin Reuptake Inhibitors may diminish the therapeutic effect of Thyroid Products. Thyroid product dose requirements may be increased. Monitor therapy
Timolol (Ophthalmic): CYP2D6 Inhibitors (Strong) may increase the serum concentration of Timolol (Ophthalmic). Monitor therapy
Tiotropium: Anticholinergic Agents may enhance the anticholinergic effect of Tiotropium. Avoid combination
Tobacco (Smoked): May diminish the therapeutic effect of OLANZapine. Tobacco (Smoked) may decrease the serum concentration of OLANZapine. Monitor therapy
Topiramate: Anticholinergic Agents may enhance the adverse/toxic effect of Topiramate. Monitor therapy
Tricyclic Antidepressants: FLUoxetine may enhance the serotonergic effect of Tricyclic Antidepressants. FLUoxetine may increase the serum concentration of Tricyclic Antidepressants. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) and increased TCA concentrations/effects if these agents are combined. Consider therapy modification
Trimeprazine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Tropisetron: CYP2D6 Inhibitors (Strong) may increase the serum concentration of Tropisetron. Monitor therapy
Umeclidinium: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Urokinase: Agents with Antiplatelet Properties may enhance the anticoagulant effect of Urokinase. Avoid combination
Valbenazine: CYP2D6 Inhibitors (Strong) may increase serum concentrations of the active metabolite(s) of Valbenazine. Monitor therapy
Valproate Products: May decrease the serum concentration of OLANZapine. Monitor therapy
Vitamin E (Systemic): May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Vitamin K Antagonists (eg, warfarin): Selective Serotonin Reuptake Inhibitors may enhance the anticoagulant effect of Vitamin K Antagonists. Monitor therapy
Voriconazole: May enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Vortioxetine: Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors) may enhance the antiplatelet effect of Vortioxetine. Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors) may enhance the serotonergic effect of Vortioxetine. This could result in serotonin syndrome. Selective Serotonin Reuptake Inhibitors (Strong CYP2D6 Inhibitors) may increase the serum concentration of Vortioxetine. Management: Consider alternatives to this drug combination. If combined, reduce the vortioxetine dose by half and monitor for signs and symptoms of bleeding and serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, autonomic instability). Consider therapy modification
Zanubrutinib: May enhance the antiplatelet effect of Agents with Antiplatelet Properties. Monitor therapy
Zolpidem: CNS Depressants may enhance the CNS depressant effect of Zolpidem. Management: Reduce the Intermezzo brand sublingual zolpidem adult dose to 1.75 mg for men who are also receiving other CNS depressants. No such dose change is recommended for women. Avoid use with other CNS depressants at bedtime; avoid use with alcohol. Consider therapy modification
Adverse Reactions
Also see individual agents.
>10%:
Cardiovascular: Edema (adults: 15%)
Central nervous system: Drowsiness (24% to 27%), fatigue (adults: 12%)
Endocrine & metabolic: Hyperprolactinemia (children and adolescents: 85%; adults: 28%), increased serum triglycerides (children and adolescents: 3% to 85%; adults: 16% to 51%), increased LDL cholesterol (children and adolescents: 13% to 75%; adults: 5% to 17%), increased serum cholesterol (children and adolescents: 4% to 73%; adults: 2% to 28%), weight gain (adults: 25% to 66%; children and adolescents: 20% to 52%), decreased HDL cholesterol (adults: 39%), decreased serum bicarbonate (14%)
Gastrointestinal: Increased appetite (17% to 20%), xerostomia (adults: 15%)
Hepatic: Increased serum ALT (children and adolescents: 46%; adults ≥3 times ULN 5%; ≥5 times ULN 2%), increased serum AST (children and adolescents: 34%), decreased serum bilirubin (adults: 15%)
1% to 10%:
Cardiovascular: Orthostatic hypotension (adults: 4%), prolonged Q-T interval on ECG (≥1%), vasodilation (≥1%)
Central nervous system: Manic reaction (adults: 7%; children and adolescents: 1%), disturbance in attention (adults: 5%), restlessness (3% to 4%), anxiety (children and adolescents: 3%), abnormality in thinking (adults: 2%), nervousness (adults: 2%), pain (adults: 2%), suicidal ideation (children and adolescents: 2%), amnesia (≥1%), chills (≥1%)
Dermatologic: Ecchymoses (≥1%), skin photosensitivity (≥1%)
Endocrine & metabolic: Glycosuria (adults: 4%), hypoalbuminemia (adults: 3%), increased uric acid (adults: 3%), hypophosphatemia (adults: 2%), hypermenorrhea (≥1%), weight loss (≥1%), menstrual disease (1%)
Gastrointestinal: Dyspepsia (children and adolescents: 3%), flatulence (adults: 3%), abdominal distension (adults: 2%), diarrhea (≥1%), dysgeusia (≥1%)
Genitourinary: Dysmenorrhea (children and adolescents: 2%), erectile dysfunction (adults: 2%), mastalgia (≥1%), urinary frequency (≥1%), urinary incontinence (≥1%)
Hematologic & oncologic: Decreased hemoglobin (adults: 3%), lymphocytopenia (adults: 2%)
Hepatic: Increased liver enzymes (children and adolescents: 9%)
Neuromuscular & skeletal: Tremor (9%), arthralgia (adults: 4%), limb pain (adults: 3%), weakness (adults: 3%), back pain (children and adolescents: 2%), stiffness (adults: 2%), neck stiffness (≥1%)
Ophthalmic: Blurred vision (adults: 5%)
Renal: Increased blood urea nitrogen (adults: 3%)
Respiratory: Sinusitis (adults: 2%)
Miscellaneous: Fever (adults: 2%)
Frequency not defined:
Cardiovascular: Bradycardia, tachycardia
Central nervous system: Anorgasmia
Endocrine & metabolic: Decreased libido, increased gamma-glutamyl transferase
Genitourinary: Ejaculatory disorder
Hepatic: Increased serum alkaline phosphatase
<1%, postmarketing, and/or case reports: Accommodation disturbance, agranulocytosis, alopecia, anaphylactoid reaction, anemia, angle-closure glaucoma, ataxia, breast hypertrophy, buccoglossal syndrome, coma, deep vein thrombosis, depersonalization, dry eye syndrome, dysarthria, dyskinesia, dystonia, emotional lability, epistaxis, euphoria, exfoliative dermatitis, galactorrhea, gastritis, gastroenteritis, gastrointestinal hemorrhage, gout, gynecomastia, hyperbilirubinemia, hyperglycemia, hyperkinesia, hypokinesia, hyponatremia, increased creatine phosphokinase, increased libido, increased serum creatinine, intestinal obstruction, lactation disorder, laryngismus, leukopenia, liver steatosis, myoclonus, nausea, neutropenia, osteoporosis, pancreatitis, peptic ulcer, pneumonitis, pruritus, pulmonary embolism, pulmonary fibrosis, purpura, rhabdomyolysis, seizure, serotonin syndrome, sexual disorder, SIADH, thrombocytopenia, urinary retention, urinary urgency, vomiting, withdrawal syndrome, xeroderma, yawning
Warnings/Precautions
Major psychiatric warnings:
- Suicidal thinking/behavior: [US Boxed Warning]: Antidepressants increase the risk of suicidal thinking and behavior in children, adolescents, and young adults in short-term studies Short-term studies did not show an increased risk in patients >24 years of age and showed a decreased risk in patients ≥65 years. Closely monitor patients for clinical worsening, and emergence of suicidal thoughts and behaviors, particularly during the initial 1 to 2 months of therapy or during periods of dosage adjustments (increases or decreases); the patient’s family or caregiver should be instructed to closely observe the patient and communicate condition with health care provider. A medication guide concerning the use of antidepressants should be dispensed with each prescription.
- The possibility of a suicide attempt is inherent in major depression and may persist until remission occurs. Worsening depression and severe abrupt suicidality that are not part of the presenting symptoms may require discontinuation or modification of drug therapy. Use caution in high-risk patients during initiation of therapy.
- Prescriptions should be written for the smallest quantity consistent with good patient care. The patient's family or caregiver should be alerted to monitor patients for the emergence of suicidality and associated behaviors such as anxiety, agitation, panic attacks, insomnia, irritability, hostility, impulsivity, akathisia, hypomania, and mania; patients should be instructed to notify their healthcare provider if any of these symptoms or worsening depression occur.
Concerns related to adverse effects:
- Allergic reactions and rash: Fluoxetine use has been associated with occurrences of significant rash and allergic reactions, including vasculitis, lupus-like syndrome, laryngospasm, anaphylactoid reactions, and pulmonary inflammatory disease. Discontinue if underlying cause of rash cannot be identified.
- Altered cardiac conduction: Olanzapine may alter cardiac conduction; life-threatening arrhythmias have occurred with therapeutic doses of antipsychotics.
- Anticholinergic effects: Olanzapine may cause anticholinergic effects (constipation, xerostomia, blurred vision, urinary retention); use with caution in patients with decreased gastrointestinal motility, urinary retention, BPH, xerostomia, or visual problems (including narrow-angle glaucoma). Relative to other neuroleptics, olanzapine has a moderate potency of cholinergic blockade (Richelson 1999).
- Bleeding risk: Fluoxetine may impair platelet aggregation resulting in increased risk of bleeding events, particularly if used concomitantly with aspirin, NSAIDs, warfarin or other anticoagulants. Bleeding related to SSRI use has been reported to range from relatively minor bruising and epistaxis to life-threatening hemorrhage.
- Blood dyscrasias: Leukopenia, neutropenia, and agranulocytosis (sometimes fatal) have been reported in clinical trials and postmarketing reports with antipsychotic use; presence of risk factors (eg, preexisting low WBC or history of drug-induced leuko-/neutropenia) should prompt periodic blood count assessment. Discontinue therapy at first signs of blood dyscrasias or if absolute neutrophil count <1000/mm3.
- Cerebrovascular effects: An increased incidence of cerebrovascular effects (eg, transient ischemic attack, stroke), including fatalities, has been reported in placebo-controlled trials of olanzapine for the unapproved use in elderly patients with dementia-related psychosis.
- CNS depression: May cause CNS depression, which may impair physical or mental abilities; patients must be cautioned about performing tasks that require mental alertness (eg, operating machinery or driving). Olanzapine may be moderate to highly sedating in comparison to other antipsychotics (APA [Lehman 2004]); dose-related effects have been observed.
- Dyslipidemia: Dose-related increases in cholesterol and triglycerides have been noted with olanzapine use. Use olanzapine with caution in patients with preexisting abnormal lipid profile.
- Esophageal dysmotility/Aspiration: Antipsychotic use has been associated with esophageal dysmotility and aspiration; risk increases with age. Use with caution in patients at risk for aspiration pneumonia (eg, Alzheimer disease), particularly in patients >75 years (Herzig 2017; Maddalena 2004).
- Extrapyramidal symptoms: Olanzapine may cause extrapyramidal symptoms (EPS), including pseudoparkinsonism, acute dystonic reactions, akathisia, and tardive dyskinesia (risk of these reactions is generally much lower relative to typical/conventional antipsychotics; frequencies are similar to placebo). Risk of dystonia (and probably other EPS) may be greater with increased doses, use of conventional antipsychotics, males, and younger patients. Factors associated with greater vulnerability to tardive dyskinesia include older in age, female gender combined with postmenopausal status, Parkinson disease, pseudoparkinsonism symptoms, affective disorders (particularly major depressive disorder), concurrent medical diseases such as diabetes, previous brain damage, alcoholism, poor treatment response, and use of high doses of antipsychotics (APA [Lehman 2004]; Soares-Weiser 2007). Consider therapy discontinuation with signs/symptoms of tardive dyskinesia.
- Falls: May increase the risk for falls due to somnolence, orthostatic hypotension and motor or sensory instability. Complete fall risk assessments at baseline and periodically during treatment in patients with diseases or on medications that may also increase fall risk.
- Fractures: Bone fractures have been associated with antidepressant treatment. Consider the possibility of a fragility fracture if an antidepressant-treated patient presents with unexplained bone pain, point tenderness, swelling, or bruising (Rabenda 2013; Rizzoli 2012).
- Hyperglycemia: Atypical antipsychotics have been associated with development of hyperglycemia; in some cases, may be extreme and associated with ketoacidosis, hyperosmolar coma, or death. Olanzapine may have a greater association with hyperglycemia than other atypical antipsychotics. Use with caution in patients with diabetes or other disorders of glucose regulation; monitor for worsening of glucose control. Patients with risk factors for diabetes (eg, obesity or family history) should have a baseline fasting blood sugar (FBS) and periodic assessment of glucose regulation.
- Hyperprolactinemia: Olanzapine may cause dose-related increases in prolactin levels; clinical significance of hyperprolactinemia in patients with breast cancer or other prolactin-dependent tumors is unknown. Clinical manifestations of increased prolactin levels included menstrual-, sexual- and breast-related events.
- Multiorgan hypersensitivity reactions (drug reaction with eosinophilia and systemic symptoms [DRESS]): Potentially serious, sometimes fatal, multiorgan hypersensitivity reactions (DRESS) have been reported with olanzapine. Symptoms may include a cutaneous reaction (rash or exfoliative dermatitis), eosinophilia, fever, and/or lymphadenopathy with systemic complications (eg, hepatitis, nephritis, pneumonitis, myocarditis, pericarditis). Discontinue olanzapine if DRESS is suspected.
- Neuroleptic malignant syndrome (NMS): Olanzapine use may be associated with NMS; monitor for mental status changes, fever, muscle rigidity, and/or autonomic instability.
- Ocular effects: May cause mild pupillary dilation which in susceptible individuals can lead to an episode of narrow-angle glaucoma. Consider evaluating patients who have not had an iridectomy for narrow-angle glaucoma risk factors.
- Orthostatic hypotension: May cause orthostatic hypotension; use with caution in patients at risk of this effect or in those who would not tolerate transient hypotensive episodes (cerebrovascular disease, cardiovascular disease, hypovolemia, or concurrent medication use which may predispose to hypotension/bradycardia).
- QT prolongation: Fluoxetine may cause QT prolongation and ventricular arrhythmia including torsade de pointes. Use with caution in patients with risk factors for QT prolongation (eg, congenital long QT syndrome, history of prolonged QT, family history of prolonged QT or sudden cardiac death), other conditions that predispose to arrhythmias (eg, hypokalemia, hypomagnesemia, recent MI, uncompensated heart failure, bradyarrhythmias or other arrhythmias, concomitant use of other agents that prolong QT interval), or increased fluoxetine exposure (eg, overdose, hepatic impairment, use of CYP2D6 inhibitors, poor CYP2D6 metabolizer status, concomitant use of other highly protein-bound drugs). Consider ECG monitoring when initiating therapy in patients with risk factors for QT prolongation and ventricular arrhythmia. Consider discontinuing therapy if ventricular arrhythmia suspected and initiate cardiac evaluation.
- Serotonin syndrome: Potentially life-threatening serotonin syndrome (SS) has occurred with serotonergic agents (eg, SSRIs, SNRIs), particularly when used in combination with other serotonergic agents (eg, triptans, TCAs, fentanyl, lithium, tramadol, buspirone, St John’s wort, tryptophan) or agents that impair metabolism of serotonin (eg, MAO inhibitors intended to treat psychiatric disorders, other MAO inhibitors [ie, linezolid and intravenous methylene blue]). Monitor patients closely for signs of SS such as mental status changes (eg, agitation, hallucinations, delirium, coma); autonomic instability (eg, tachycardia, labile blood pressure, diaphoresis); neuromuscular changes (eg, tremor, rigidity, myoclonus); GI symptoms (eg, nausea, vomiting, diarrhea); and/or seizures. Discontinue treatment (and any concomitant serotonergic agent) immediately if signs/symptoms arise.
- Sexual dysfunction: May cause or exacerbate sexual dysfunction.
- SIADH and hyponatremia: SSRIs and SNRIs have been associated with the development of SIADH; hyponatremia has been reported rarely (including severe cases with serum sodium <110 mmol/L), predominately in the elderly. Volume depletion and/or concurrent use of diuretics likely increases risk. Consider discontinuation if symptomatic hyponatremia occurs.
- Temperature regulation: Impaired core body temperature regulation may occur; caution with strenuous exercise, heat exposure, dehydration, and concomitant medication possessing anticholinergic effects.
- Weight gain: Significant weight gain (≥7% of baseline weight) has been observed with antipsychotic therapy; incidence varies with product. Dose-related changes have been observed with olanzapine. Monitor waist circumference and BMI.
Disease-related concerns:
- Cardiovascular disease: Use with caution in patients with severe cardiac disease, hemodynamic instability, prior myocardial infarction, ischemic heart disease, or hypercholesterolemia.
- Dementia: [US Boxed Warning]: Elderly patients with dementia-related behavioral disorders treated with antipsychotics are at an increased risk of death compared to placebo. Most deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Olanzapine and fluoxetine are not approved for the treatment of dementia-related psychosis.
- Hepatic impairment: Use with caution in patients with hepatic disease or impairment; lowest starting dose recommended; may increase transaminases (primarily ALT).
- Mania/hypomania: May worsen psychosis in some patients or precipitate a shift to mania or hypomania in patients with bipolar disorder. Monotherapy in patients with bipolar disorder should be avoided. Patients presenting with depressive symptoms should be screened for bipolar disorder. This combination is FDA approved for the treatment of depressive episodes associated with bipolar disorder.
- Renal impairment: Use with caution in patients with renal disease; a lower dosage may be needed.
- Seizure disorder: Use with caution in patients at risk of seizures, including those with a history of seizures, head trauma, brain damage, alcoholism, or concurrent therapy with medications which may lower seizure threshold, or conditions that potentially lower the seizure threshold (eg Alzheimer dementia). Elderly patients may be at increased risk of seizures due to an increased prevalence of predisposing factors.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Smokers: Olanzapine levels may be lower in patients who smoke.
Other warnings/precautions:
- Antidepressant discontinuation syndrome: Abrupt discontinuation or interruption of antidepressant therapy has been associated with a discontinuation syndrome. Symptoms arising may vary with antidepressant however commonly include nausea, vomiting, diarrhea, headaches, lightheadedness, dizziness, diminished appetite, sweating, chills, tremors, paresthesias, fatigue, somnolence, and sleep disturbances (eg, vivid dreams, insomnia). Less common symptoms include electric shock-like sensations, cardiac arrhythmias (more common with tricyclic antidepressants), myalgias, parkinsonism, arthralgias, and balance difficulties. Psychological symptoms may also emerge such as agitation, anxiety, akathisia, panic attacks, irritability, aggressiveness, worsening of mood, dysphoria, mood lability, hyperactivity, mania/hypomania, depersonalization, decreased concentration, slowed thinking, confusion, and memory or concentration difficulties. Greater risks for developing a discontinuation syndrome have been associated with antidepressants with shorter half-lives, longer durations of treatment, and abrupt discontinuation. For antidepressants of short or intermediate half-lives, symptoms may emerge within 2 to 5 days after treatment discontinuation and last 7 to 14 days (APA, 2010; Fava, 2006; Haddad, 2001; Shelton, 2001; Warner, 2006).
- Discontinuation of therapy: When discontinuing antipsychotic therapy, the American Psychiatric Association (APA), Canadian Psychiatric Association (CPA), and World Federation of Societies of Biological Psychiatry (WFSBP) guidelines recommend gradually tapering antipsychotics to avoid physical withdrawal symptoms, including anorexia, anxiety, diaphoresis, diarrhea, dizziness, dyskinesia, headache, myalgia, nausea, paresthesia, restlessness, tremulousness, and vomiting (APA [Lehman 2004]; CPA [Addington 2005]; Lambert 2007; WFSBP [Hasan 2012]). The risk of withdrawal symptoms is highest following abrupt discontinuation of highly anti-cholinergic or dopaminergic antipsychotics (Cerovecki 2013). Additional factors such as duration of antipsychotic exposure, the indication for use, medication half-life, and risk for relapse should be considered In schizophrenia, there is no reliable indicator to differentiate the minority who will not from the majority who will relapse with drug discontinuation. However, studies in which the medication of well-stabilized patients were discontinued indicate that 75% of patients relapse within 6 to 24 months. Indefinite maintenance antipsychotic medication is generally recommended, and especially for patients who have had multiple prior episodes or 2 episodes within 5 years (APA [Lehman 2004]).
- Electroconvulsive therapy: May increase the risks associated with electroconvulsive therapy; consider discontinuing, when possible, prior to ECT treatment.
- Long half-life: Due to the long half-life of fluoxetine and its metabolites, the effects and interactions noted may persist for prolonged periods following discontinuation.
Monitoring Parameters
Vital signs (as clinically indicated); blood pressure (baseline; repeat 3 months after antipsychotic initiation, then yearly); fasting lipid panel (baseline; repeat 3 months after initiation of antipsychotic; if LDL level is normal repeat at 2 to 5 year intervals or more frequently if clinical indicated); fasting blood glucose/HbA1c (baseline; repeat 3 months after starting antipsychotic, then yearly); weight; height; BMI; waist circumference (baseline; repeat at 4, 8, and 12 weeks after initiating or changing therapy, then quarterly; consider switching to a different antipsychotic for a weight gain ≥5% of initial weight); personal and family history of obesity, diabetes, dyslipidemia, hypertension, or cardiovascular disease (baseline; repeat annually); mental status; CBC (as clinically indicated; monitor frequently during the first few months of therapy in patients with preexisting low WBC or history of drug-induced leukopenia/neutropenia); changes in menstruation, libido, development of galactorrhea, erectile and ejaculatory function (at each visit for the first 12 weeks after the antipsychotic is initiated or until the dose is stable, then yearly); abnormal involuntary movements or parkinsonian signs (baseline; repeat weekly until dose stabilized for at least 2 weeks after introduction and for 2 weeks after any significant dose increase); tardive dyskinesia (every 12 months; high-risk patients every 6 months); ocular examination (yearly in patients >40 years; every 2 years in younger patients); signs and symptoms of depression, anxiety, suicidal ideation (especially at the beginning of therapy or when doses are increased or decreased), sleep; signs/symptoms of serotonin syndrome; electrolytes and liver function (annually and as clinically indicated); serum sodium in at-risk populations (as clinically indicated) (ADA 2004; Lehman 2004; Marder 2004).
Pregnancy
Pregnancy Risk Factor
C
Pregnancy Considerations
Adverse events were observed in animal reproduction studies using this combination. Refer to individual agents for additional information.
Patient Education
What is this drug used for?
- It is used to treat low mood (depression).
- It is used to treat bipolar problems.
Frequently reported side effects of this drug
- Dry mouth
- Loss of strength and energy
- Fatigue
- Increased appetite
- Weight gain
- Tremors
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Infection
- Depression like thoughts of suicide, anxiety, agitation, irritability, panic attacks, mood changes, behavioral changes, or confusion
- High blood sugar like confusion, fatigue, more thirst, hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit
- Low sodium like headache, trouble focusing, memory problems, confusion, weakness, seizures, or change in balance
- Serotonin syndrome like dizziness, severe headache, agitation, sensing things that seem real but are not, fast heartbeat, abnormal heartbeat, flushing, tremors, sweating a lot, change in balance, severe nausea, or severe diarrhea
- Neuroleptic malignant syndrome like fever, muscle cramps or stiffness, dizziness, severe headache, confusion, change in thinking, fast heartbeat, abnormal heartbeat, or sweating a lot
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin
- Tardive dyskinesia like unable to control body movements; tongue, face, mouth, or jaw sticking out; mouth puckering; or puffing cheeks
- Bruising
- Bleeding
- Severe dizziness
- Passing out
- Swelling
- Enlarged breasts
- Nipple discharge
- Sexual dysfunction
- No menstrual periods
- Lack of sweating
- Difficult urination
- Change in amount of urine passed
- Swollen glands
- Eye pain
- Vision changes
- Eye swelling
- Eye redness
- Fast heartbeat
- Abnormal heartbeat
- Shortness of breath
- Erection that lasts more than 4 hours
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.