Boxed Warning
Fetal toxicity:
When pregnancy is detected, discontinue olmesartan/hydrochlorothiazide as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
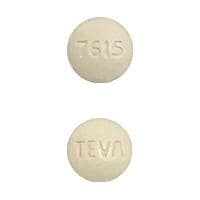
Benicar HCT 20/12.5: Olmesartan 20 mg and hydrochlorothiazide 12.5 mg
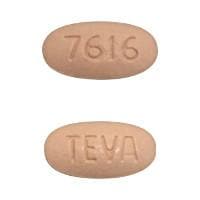
Benicar HCT 40/12.5: Olmesartan medoxomil 40 mg and hydrochlorothiazide 12.5 mg
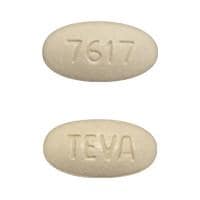
Benicar HCT 40/25: Olmesartan medoxomil 40 mg and hydrochlorothiazide 25 mg
Generic: 20/12.5: Olmesartan 20 mg and hydrochlorothiazide 12.5 mg; 40/12.5: Olmesartan 40 mg and hydrochlorothiazide 12.5 mg; 40/25: Olmesartan 40 mg and hydrochlorothiazide 25 mg
Pharmacology
Mechanism of Action
Olmesartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II. Hydrochlorothiazide inhibits sodium reabsorption in the distal tubules causing increased excretion of sodium and water as well as potassium and hydrogen ions.
Use: Labeled Indications
Hypertension: Management of hypertension (not indicated for initial treatment)
Contraindications
Hypersensitivity to olmesartan, hydrochlorothiazide, or any component of the formulation; concomitant use with aliskiren in patients with diabetes mellitus; anuria
Note: Although some product labeling states this medication is contraindicated with other sulfonamide-containing drug classes, the scientific basis of this statement has been challenged. See “Warnings/Precautions” for more detail.
Documentation of allergenic cross-reactivity for angiotensin II receptor blockers and thiazide-related diuretics is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Canadian labeling: Additional contraindications (not in US labeling): Hypersensitivity to other sulfonamide-derived drugs; concomitant use with aliskiren in patients with moderate to severe renal impairment (GFR <60 mL/minute/1.73 m2)
Dosage and Administration
Dosing: Adult
Hypertension: Oral:
Replacement therapy: Combination product may be substituted for individual titrated agents.
Initiation of combination therapy when monotherapy has failed to achieve desired effects:
Patients currently on olmesartan monotherapy: Initial: Olmesartan 40 mg/hydrochlorothiazide 12.5 mg once daily; may titrate dose after 2 to 4 weeks (maximum: olmesartan 40 mg/hydrochlorothiazide 25 mg per day).
Patients currently on hydrochlorothiazide monotherapy: Initial: Olmesartan 20 mg/hydrochlorothiazide 12.5 mg once daily; may titrate dose after 2 to 4 weeks (maximum: olmesartan 40 mg/hydrochlorothiazide 25 mg per day).
Dosing: Geriatric
Refer to adult dosing.
Administration
Oral: Administer with or without food.
Storage
Store at 20°C to 25°C (68°F to 77°F).
Olmesartan and Hydrochlorothiazide Images
Drug Interactions
Ajmaline: Sulfonamides may enhance the adverse/toxic effect of Ajmaline. Specifically, the risk for cholestasis may be increased. Monitor therapy
Alcohol (Ethyl): May enhance the orthostatic hypotensive effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Aliskiren: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Aliskiren may enhance the hypotensive effect of Angiotensin II Receptor Blockers. Aliskiren may enhance the nephrotoxic effect of Angiotensin II Receptor Blockers. Management: Aliskiren use with ACEIs or ARBs in patients with diabetes is contraindicated. Combined use in other patients should be avoided, particularly when CrCl is less than 60 mL/min. If combined, monitor potassium, creatinine, and blood pressure closely. Consider therapy modification
Allopurinol: Thiazide and Thiazide-Like Diuretics may enhance the potential for allergic or hypersensitivity reactions to Allopurinol. Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Allopurinol. Specifically, Thiazide Diuretics may increase the concentration of Oxypurinol, an active metabolite of Allopurinol. Monitor therapy
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Aminolevulinic Acid (Systemic): Photosensitizing Agents may enhance the photosensitizing effect of Aminolevulinic Acid (Systemic). Avoid combination
Aminolevulinic Acid (Topical): Photosensitizing Agents may enhance the photosensitizing effect of Aminolevulinic Acid (Topical). Monitor therapy
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Angiotensin II: Receptor Blockers may diminish the therapeutic effect of Angiotensin II. Monitor therapy
Angiotensin-Converting Enzyme Inhibitors: Angiotensin II Receptor Blockers may enhance the adverse/toxic effect of Angiotensin-Converting Enzyme Inhibitors. Angiotensin II Receptor Blockers may increase the serum concentration of Angiotensin-Converting Enzyme Inhibitors. Management: In US labeling, use of telmisartan and ramipril is not recommended. It is not clear if any other combination of an ACE inhibitor and an ARB would be any safer. Consider alternatives to the combination when possible. Consider therapy modification
Anticholinergic Agents: May increase the serum concentration of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Antidiabetic Agents: Thiazide and Thiazide-Like Diuretics may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Beta2-Agonists: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Bile Acid Sequestrants: May decrease the absorption of Thiazide and Thiazide-Like Diuretics. The diuretic response is likewise decreased. Consider therapy modification
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Calcium Salts: Thiazide and Thiazide-Like Diuretics may decrease the excretion of Calcium Salts. Continued concomitant use can also result in metabolic alkalosis. Monitor therapy
CarBAMazepine: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of CarBAMazepine. Specifically, there may be an increased risk for hyponatremia. Monitor therapy
Cardiac Glycosides: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Cardiac Glycosides. Specifically, cardiac glycoside toxicity may be enhanced by the hypokalemic and hypomagnesemic effect of thiazide diuretics. Monitor therapy
Colesevelam: May decrease the serum concentration of Olmesartan. Management: Administer olmesartan at least 4 hours prior to colesevelam. Consider therapy modification
Corticosteroids (Orally Inhaled): May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Corticosteroids (Systemic): May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Cyclophosphamide: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Cyclophosphamide. Specifically, granulocytopenia may be enhanced. Monitor therapy
CycloSPORINE (Systemic): Angiotensin II Receptor Blockers may enhance the hyperkalemic effect of CycloSPORINE (Systemic). Monitor therapy
Dapoxetine: May enhance the orthostatic hypotensive effect of Angiotensin II Receptor Blockers. Monitor therapy
Dexketoprofen: May enhance the adverse/toxic effect of Sulfonamides. Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diacerein: May enhance the therapeutic effect of Diuretics. Specifically, the risk for dehydration or hypokalemia may be increased. Monitor therapy
Diazoxide: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Diazoxide. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Dichlorphenamide: Thiazide and Thiazide-Like Diuretics may enhance the hypokalemic effect of Dichlorphenamide. Monitor therapy
Dofetilide: HydroCHLOROthiazide may enhance the QTc-prolonging effect of Dofetilide. HydroCHLOROthiazide may increase the serum concentration of Dofetilide. Avoid combination
Drospirenone: Angiotensin II Receptor Blockers may enhance the hyperkalemic effect of Drospirenone. Monitor therapy
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
Eltrombopag: May increase the serum concentration of OATP1B1/1B3 (SLCO1B1/1B3) Substrates. Monitor therapy
Eplerenone: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Fexinidazole [INT]: Thiazide and Thiazide-Like Diuretics may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Gemfibrozil: May increase the serum concentration of OATP1B1/1B3 (SLCO1B1/1B3) Substrates. See separate drug interaction monographs for agents listed as exceptions. Monitor therapy
Heparin: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Heparins (Low Molecular Weight): May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Ipragliflozin: May enhance the adverse/toxic effect of Thiazide and Thiazide-Like Diuretics. Specifically, the risk for intravascular volume depletion may be increased. Monitor therapy
Ivabradine: Thiazide and Thiazide-Like Diuretics may enhance the arrhythmogenic effect of Ivabradine. Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Levosulpiride: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Levosulpiride. Avoid combination
Licorice: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Lithium: Thiazide and Thiazide-Like Diuretics may decrease the excretion of Lithium. Consider therapy modification
Lithium: Angiotensin II Receptor Blockers may increase the serum concentration of Lithium. Management: Lithium dosage reductions will likely be needed following the addition of an angiotensin II receptor antagonist. Consider therapy modification
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Mecamylamine: Sulfonamides may enhance the adverse/toxic effect of Mecamylamine. Avoid combination
Methenamine: Thiazide and Thiazide-Like Diuretics may diminish the therapeutic effect of Methenamine. Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Multivitamins/Fluoride (with ADE): May enhance the hypercalcemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Multivitamins/Minerals (with ADEK, Folate, Iron): Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Multivitamins/Minerals (with ADEK, Folate, Iron). Monitor therapy
Multivitamins/Minerals (with AE, No Iron): Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Multivitamins/Minerals (with AE, No Iron). Specifically, thiazide diuretics may decrease the excretion of calcium, and continued concomitant use can also result in metabolic alkalosis. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Neuromuscular-Blocking Agents (Nondepolarizing): Thiazide and Thiazide-Like Diuretics may enhance the neuromuscular-blocking effect of Neuromuscular-Blocking Agents (Nondepolarizing). Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: Thiazide and Thiazide-Like Diuretics may enhance the nephrotoxic effect of Nonsteroidal Anti-Inflammatory Agents. Nonsteroidal Anti-Inflammatory Agents may diminish the therapeutic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: Angiotensin II Receptor Blockers may enhance the adverse/toxic effect of Nonsteroidal Anti-Inflammatory Agents. Specifically, the combination may result in a significant decrease in renal function. Nonsteroidal Anti-Inflammatory Agents may diminish the therapeutic effect of Angiotensin II Receptor Blockers. The combination of these two agents may also significantly decrease glomerular filtration and renal function. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Opioid Agonists: May enhance the adverse/toxic effect of Diuretics. Opioid Agonists may diminish the therapeutic effect of Diuretics. Monitor therapy
OXcarbazepine: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of OXcarbazepine. Specifically, there may be an increased risk for hyponatremia. Monitor therapy
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Porfimer: Photosensitizing Agents may enhance the photosensitizing effect of Porfimer. Monitor therapy
Potassium Salts: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Potassium-Sparing Diuretics: Angiotensin II Receptor Blockers may enhance the hyperkalemic effect of Potassium-Sparing Diuretics. Monitor therapy
Promazine: Thiazide and Thiazide-Like Diuretics may enhance the QTc-prolonging effect of Promazine. Avoid combination
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Ranolazine: May enhance the adverse/toxic effect of Angiotensin II Receptor Blockers. Monitor therapy
Reboxetine: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Selective Serotonin Reuptake Inhibitors: May enhance the hyponatremic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Sodium Phosphates: Angiotensin II Receptor Blockers may enhance the nephrotoxic effect of Sodium Phosphates. Specifically, the risk of acute phosphate nephropathy may be enhanced. Management: Consider avoiding this combination by temporarily suspending treatment with ARBs, or seeking alternatives to oral sodium phosphate bowel preparation. If the combination cannot be avoided, maintain adequate hydration and monitor renal function closely. Consider therapy modification
Sodium Phosphates: Diuretics may enhance the nephrotoxic effect of Sodium Phosphates. Specifically, the risk of acute phosphate nephropathy may be enhanced. Management: Consider avoiding this combination by temporarily suspending treatment with diuretics, or seeking alternatives to oral sodium phosphate bowel preparation. If the combination cannot be avoided, hydrate adequately and monitor fluid and renal status. Consider therapy modification
Tacrolimus (Systemic): Angiotensin II Receptor Blockers may enhance the hyperkalemic effect of Tacrolimus (Systemic). Monitor therapy
Teriflunomide: May increase the serum concentration of OATP1B1/1B3 (SLCO1B1/1B3) Substrates. Monitor therapy
Tolvaptan: May increase the serum concentration of OATP1B1/1B3 (SLCO1B1/1B3) Substrates. Consider therapy modification
Topiramate: Thiazide and Thiazide-Like Diuretics may enhance the hypokalemic effect of Topiramate. Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Topiramate. Management: Monitor for increased topiramate levels/adverse effects (e.g., hypokalemia) with initiation/dose increase of a thiazide diuretic. Closely monitor serum potassium concentrations with concomitant therapy. Topiramate dose reductions may be necessary. Consider therapy modification
Toremifene: Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Toremifene. Monitor therapy
Trimethoprim: May enhance the hyperkalemic effect of Angiotensin II Receptor Blockers. Monitor therapy
Valsartan: HydroCHLOROthiazide may enhance the hypotensive effect of Valsartan. Valsartan may increase the serum concentration of HydroCHLOROthiazide. Monitor therapy
Verteporfin: Photosensitizing Agents may enhance the photosensitizing effect of Verteporfin. Monitor therapy
Vitamin D Analogs: Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Vitamin D Analogs. Monitor therapy
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
See individual agents.
Adverse Reactions
Adverse reactions reported with combination product. Also see individual agents.
1% to 10%:
Central nervous system: Dizziness (9%)
Endocrine & metabolic: Hyperuricemia (4%)
Gastrointestinal: Nausea (3%)
Respiratory: Upper respiratory tract infection (7%)
Frequency not defined:
Cardiovascular: Chest pain, peripheral edema
Central nervous system: Vertigo
Dermatologic: Skin rash
Endocrine & metabolic: Hyperglycemia, hyperlipidemia
Gastrointestinal: Abdominal pain, diarrhea, dyspepsia, gastroenteritis
Genitourinary: Hematuria
Hepatic: Increased serum transaminases
Neuromuscular & skeletal: Back pain, arthralgia, arthritis, increased creatine phosphokinase, myalgia
Respiratory: Cough
<1%, postmarketing, and/or case reports: Acute renal failure, alopecia, angioedema, anaphylaxis, facial edema, hyperkalemia, increased blood urea nitrogen, increased serum creatinine, pruritus, rhabdomyolysis, urticaria, vomiting, weakness
Warnings/Precautions
Concerns related to adverse effects:
- Angioedema: Angioedema has been reported rarely with some angiotensin II receptor antagonists (ARBs) and may occur at any time during treatment (especially following first dose). It may involve the head and neck (potentially compromising airway) or the intestine (presenting with abdominal pain). Patients with idiopathic or hereditary angioedema or previous angioedema associated with ACE-inhibitor therapy may be at an increased risk. Prolonged frequent monitoring may be required, especially if tongue, glottis, or larynx are involved, as they are associated with airway obstruction. Patients with a history of airway surgery may have a higher risk of airway obstruction. Discontinue therapy immediately if angioedema occurs. Aggressive early management is critical. Intramuscular (IM) administration of epinephrine may be necessary. Do not readminister to patients who have had angioedema with ARBs.
- Electrolyte disturbances: Hyperkalemia may occur with angiotensin II receptor antagonists; risk factors include renal dysfunction, diabetes mellitus, and concomitant use of potassium-sparing diuretics, potassium supplements, and/or potassium-containing salts. Use cautiously, if at all, with these agents and monitor potassium closely. Thiazide diuretics may cause hypokalemia, hypochloremic alkalosis, hypomagnesemia, and hyponatremia.
- Gastrointestinal effects: Symptoms of sprue-like enteropathy (ie, severe, chronic diarrhea with significant weight loss) has been reported with olmesartan; may develop years after treatment initiation with villous atrophy commonly found on intestinal biopsy. Once other etiologies have been excluded, discontinue treatment and consider other antihypertensive treatment. Clinical and histologic improvement was noted after treatment was discontinued in a case series of 22 patients (Rubio-Tapia 2012).
- Gout: In certain patients with a history of gout, a familial predisposition to gout, or chronic renal failure, gout can be precipitated by hydrochlorothiazide. This risk may be increased with doses ≥25 mg (Gurwitz 1997).
- Hypersensitivity reactions: Hypersensitivity reactions may occur with hydrochlorothiazide. Risk is increased in patients with a history of allergy or bronchial asthma.
- Hypotension: Symptomatic hypotension may occur upon initiation in patients who are salt- or volume-depleted (eg, those treated with high-dose diuretics); correct volume depletion prior to administration. This transient hypotensive response is not a contraindication to further treatment with olmesartan/hydrochlorothiazide.
- Ocular effects: Hydrochlorothiazide may cause acute transient myopia and acute angle-closure glaucoma, typically occurring within hours to weeks following initiation; discontinue therapy immediately in patients with acute decreases in visual acuity or ocular pain. Additional treatments may be needed if uncontrolled intraocular pressure persists. Risk factors may include a history of sulfonamide or penicillin allergy.
- Photosensitivity: May occur with hydrochlorothiazide.
- Renal function deterioration: May be associated with deterioration of renal function and/or increases in serum creatinine, particularly in patients with low renal blood flow (eg, renal artery stenosis, heart failure) whose glomerular filtration rate (GFR) is dependent on efferent arteriolar vasoconstriction by angiotensin II; deterioration may result in oliguria, acute renal failure, and progressive azotemia. Small increases in serum creatinine may occur following initiation; consider discontinuation only in patients with progressive and/or significant deterioration in renal function.
- Sulfonamide (“sulfa”) allergy: The FDA-approved product labeling for many medications containing a sulfonamide chemical group includes a broad contraindication in patients with a prior allergic reaction to sulfonamides. There is a potential for cross-reactivity between members of a specific class (eg, two antibiotic sulfonamides). However, concerns for cross-reactivity have previously extended to all compounds containing the sulfonamide structure (SO2NH2). An expanded understanding of allergic mechanisms indicates cross-reactivity between antibiotic sulfonamides and nonantibiotic sulfonamides may not occur or at the very least this potential is extremely low (Brackett 2004; Johnson 2005; Slatore 2004; Tornero 2004). In particular, mechanisms of cross-reaction due to antibody production (anaphylaxis) are unlikely to occur with nonantibiotic sulfonamides. T-cell-mediated (type IV) reactions (eg, maculopapular rash) are less well understood and it is not possible to completely exclude this potential based on current insights. In cases where prior reactions were severe (Stevens-Johnson syndrome/TEN), some clinicians choose to avoid exposure to these classes.
Disease-related concerns:
- Aortic/mitral stenosis: Use olmesartan with caution in patients with significant aortic/mitral stenosis.
- Bariatric surgery: Dehydration: Avoid diuretics in the immediate postoperative period after bariatric surgery; electrolyte disturbances and dehydration may occur. Diuretics may be resumed, if indicated, once oral fluid intake goals are met (Ziegler 2009).
- Diabetes: Use hydrochlorothiazide with caution in patients with prediabetes or diabetes mellitus; may see a change in glucose control.
- Hepatic impairment: Use caution in patients with severe hepatic impairment. In progressive or severe hepatic disease, avoid electrolyte and acid/base imbalances that might lead to hepatic encephalopathy/coma.
- Hypercalcemia: Thiazide diuretics may decrease renal calcium excretion; consider avoiding use in patients with hypercalcemia.
- Hypercholesterolemia: Use with caution in patients with moderate or high cholesterol concentrations; increased cholesterol and triglyceride levels have been reported with thiazides.
- Parathyroid disease: Thiazide diuretics reduce calcium excretion; pathologic changes in the parathyroid glands with hypercalcemia and hypophosphatemia have been observed with prolonged use; should be discontinued prior to testing for parathyroid function.
- Renal artery stenosis: Use olmesartan with caution in patients with unstented unilateral/bilateral renal artery stenosis. When unstented bilateral renal artery stenosis is present, use is generally avoided due to the elevated risk of deterioration in renal function unless possible benefits outweigh risks.
- Renal impairment: Use with caution in patients with renal impairment. Cumulative effects of hydrochlorothiazide may develop, including azotemia, in patients with impaired renal function. Avoid hydrochlorothiazide in severe renal disease (ineffective). Contraindicated in patients with anuria.
- Systemic lupus erythematosus (SLE): Hydrochlorothiazide can cause SLE exacerbation or activation.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pregnancy: [US Boxed Warning]: Drugs that act on the renin-angiotensin system can cause injury and death to the developing fetus. Discontinue as soon as possible once pregnancy is detected.
- Surgical patients: In patients on chronic angiotensin receptor blocker (ARB) therapy, intraoperative hypotension may occur with induction and maintenance of general anesthesia; however, discontinuation of therapy prior to surgery is controversial. If continued preoperatively, avoidance of hypotensive agents during surgery is prudent (Hillis 2011).
Monitoring Parameters
Blood pressure, serum electrolytes, BUN, creatinine
Pregnancy
Pregnancy Risk Factor
D
Pregnancy Considerations
[US Boxed Warning]: Drugs that act on the renin-angiotensin system can cause injury and death to the developing fetus. Discontinue as soon as possible once pregnancy is detected. Also see individual agents.
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience dizziness, stuffy nose, cough, or runny nose. Have patient report immediately to prescriber signs of high blood sugar (confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit), signs of kidney problems (unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain), signs of fluid and electrolyte problems (mood changes, confusion, muscle pain or weakness, abnormal heartbeat, severe dizziness, passing out, fast heartbeat, increased thirst, seizures, loss of strength and energy, lack of appetite, unable to pass urine or change in amount of urine passed, dry mouth, dry eyes, or nausea or vomiting), signs of pancreatitis (severe abdominal pain, severe back pain, severe nausea, or vomiting), signs of lupus (rash on the cheeks or other body parts, sunburn easy, muscle or joint pain, chest pain or shortness of breath, or swelling in the arms or legs), chest pain, yellow skin, chills, swelling of arms or legs, severe or persistent diarrhea, excessive weight loss, sore throat, severe loss of strength and energy, bruising, bleeding, agitation, skin changes, vision changes, or eye pain (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.