Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Generic: 500 mg
Pharmacology
Mechanism of Action
Competitively inhibits the reabsorption of uric acid at the proximal convoluted tubule, thereby promoting its excretion and reducing serum uric acid levels; increases plasma levels of weak organic acids (penicillins, cephalosporins, or other beta-lactam antibiotics) by competitively inhibiting their renal tubular secretion
Pharmacokinetics/Pharmacodynamics
Absorption
Rapid and complete
Metabolism
Hepatic
Excretion
Urine
Onset of Action
Effect on penicillin levels: 2 hours; Uric acid renal clearance: 30 minutes
Time to Peak
Serum: 2 to 4 hours
Half-Life Elimination
Dose dependent: Normal renal function: 6 to 12 hours
Protein Binding
85% to 95%
Use: Labeled Indications
Treatment of hyperuricemia associated with gout or gouty arthritis; prolongation and elevation of beta-lactam plasma levels
Guideline recommendations:
Gout: Uricosurics, such as probenecid, are recommended alone or in combination with allopurinol in patients without proper control with allopurinol alone (EULAR [Richette 2016]).
Use: Off Label
Neurosyphilis, including ocular syphilis yes
Based on the Centers for Disease Control and Prevention (CDC) sexually transmitted diseases treatment guidelines, probenecid in combination with procaine penicillin G is an effective and recommended alternative regimen in the treatment neurosyphilis, including ocular syphilis.
Pelvic inflammatory diseaseyes
Based on the CDC sexually transmitted diseases treatment guidelines, probenecid (in combination with cefoxitin plus doxycycline with or without metronidazole) is an effective and recommended regimen for the treatment of mild to moderately severe acute pelvic inflammatory disease.
Contraindications
Hypersensitivity to probenecid or any component of the formulation; small- or large-dose aspirin therapy; blood dyscrasias; uric acid kidney stones; children <2 years of age; initiation during an acute gout attack
Dosage and Administration
Dosing: Adult
Hyperuricemia with gout: Oral: 250 mg twice daily for 1 week; may increase to 500 mg twice daily; if needed, may increase to a maximum of 2 g/day (increase dosage in 500 mg increments every 4 weeks). If serum uric acid levels are within normal limits and gout attacks have been absent for 6 months, daily dosage may be reduced by 500 mg every 6 months.
Prolong penicillin serum levels: Oral: 500 mg 4 times/day. Note: Dosing per manufacturer, see indication-specific dosing.
Neurosyphilis, including ocular syphilis (alternative to preferred therapy) (off-label use): Oral: 500 mg 4 times/day plus procaine penicillin IM for 10 to 14 days (CDC [Workowski 2015]).
Pelvic inflammatory disease (off-label use): Oral: 1 g as a single dose in combination with cefoxitin IM as a single dose plus oral doxycycline (with or without oral metronidazole) to complete 14 days of therapy (CDC [Workowski 2015])
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Prolongation of penicillin serum levels: Children ≥2 years and Adolescents: Note: Dosing for some indication-specific dosing may vary.
Patient weight ≤50 kg: Oral: Initial: 25 mg/kg/dose or 700 mg/m2/dose as a single dose; maintenance: 40 mg/kg/day or 1,200 mg /m2/day in 4 divided doses; maximum dose: 500 mg.
Patient weight >50 kg: Oral: 500 mg 4 times daily.
Gonorrhea, uncomplicated infections of cervix, urethra, and rectum: Adolescents >45 kg: Oral: 1,000 mg as a single dose with cefoxitin; Note: Combination of ceftriaxone and azithromycin is preferred (CDC [Workowski 2015]).
Pelvic inflammatory disease: Adolescents: Oral: 1,000 mg as a single dose with cefoxitin in combination with doxycycline with/without metronidazole (CDC [Workowski 2015]).
Neurosyphilis: Adolescents: Oral: 500 mg 4 times daily with procaine penicillin for 10 to 14 days; alternative therapy to aqueous penicillin G therapy (CDC [Workowski 2015]).
Cidofovir nephrotoxicity, prevention: Limited data available; various regimens have been reported (Anderson 2008; Bhadri 2009; Cesaro 2005; Doan 2007; Williams 2009; Yusuf 2006):
Infants, Children, and Adolescents: Note: The manufacturer's labeling considers use in patients <2 years of age to be contraindicated; however, clinical studies have included younger ages (including infants) for this indication.
Weight-based dosing: Oral: 25 to 40 mg/kg/dose (maximum dose: 2,000 mg) administered 3 hours before cidofovir infusion and 10 to 20 mg/kg/dose (maximum dose: 1,000 mg) at 2 to 3 hours and 8 to 9 hours after cidofovir infusion.
BSA-based dosing: Oral: 1,000 or 1,250 mg/m2/dose administered 3 hours prior to cidofovir, followed by 500 to 1,250 mg/m2/dose 1 to 2 hours and 8 hours after completion.
Administration
Administer with food to minimize GI effects.
Dietary Considerations
Drug may cause GI upset; take with food if GI upset. Drink plenty of fluids.
Storage
Store at 20°C to 25°C (68°F to 77°F). Protect from light.
Probenecid Images
Drug Interactions
Acetaminophen: Probenecid may increase the serum concentration of Acetaminophen. Probenecid may also limit the formation of at least one major non-toxic metabolite, possibly increasing the potential for formation of the toxic NAPQI metabolite. Consider therapy modification
Anagliptin: Probenecid may increase the serum concentration of Anagliptin. Monitor therapy
Avibactam: Probenecid may increase the serum concentration of Avibactam. Avoid combination
Baricitinib: Probenecid may increase the serum concentration of Baricitinib. Management: Decrease the dose of baricitinib to 1 mg daily when combined with probenecid. Consider therapy modification
Betalactamase Inhibitors: Probenecid may increase the serum concentration of Betalactamase Inhibitors. Management: Coadministration of probenecid with amoxicillin/clavulanate is not recommended per official package labeling. Exceptions: Avibactam; Sulbactam. Consider therapy modification
Cabozantinib: MRP2 Inhibitors may increase the serum concentration of Cabozantinib. Monitor therapy
Cefotaxime: Probenecid may increase the serum concentration of Cefotaxime. Management: Avoid cefotaxime doses greater than 6 g/day with concurrent probenecid. Any patients receiving this combination should be monitored closely for evidence of cefotaxime toxicity. Consider therapy modification
Cephalosporins: Probenecid may increase the serum concentration of Cephalosporins. Monitor therapy
Dapsone (Systemic): Probenecid may increase the serum concentration of Dapsone (Systemic). Monitor therapy
Deferiprone: UGT1A6 Inhibitors may increase the serum concentration of Deferiprone. Avoid combination
Dexketoprofen: Probenecid may increase the serum concentration of Dexketoprofen. Monitor therapy
Dichlorphenamide: OAT1/3 Inhibitors may increase the serum concentration of Dichlorphenamide. Monitor therapy
Doripenem: Probenecid may increase the serum concentration of Doripenem. This effect is due to probenecid's ability to decrease the active tubular secretion of doripenem. Avoid combination
Ertapenem: Probenecid may increase the serum concentration of Ertapenem. Monitor therapy
Ganciclovir-Valganciclovir: Probenecid may increase the serum concentration of Ganciclovir-Valganciclovir. Monitor therapy
Gemifloxacin: Probenecid may increase the serum concentration of Gemifloxacin. Monitor therapy
Imipenem: Probenecid may increase the serum concentration of Imipenem. Monitor therapy
Ketoprofen: Probenecid may increase the serum concentration of Ketoprofen. Monitor therapy
Ketorolac (Nasal): Probenecid may increase the serum concentration of Ketorolac (Nasal). Avoid combination
Ketorolac (Systemic): Probenecid may increase the serum concentration of Ketorolac (Systemic). Avoid combination
Loop Diuretics: Probenecid may enhance the adverse/toxic effect of Loop Diuretics. Probenecid may diminish the diuretic effect of Loop Diuretics. Probenecid may increase the serum concentration of Loop Diuretics. Management: Monitor for decreased diuretic effects or increased adverse effects of loop diuretics with concomitant use of probenecid. Bumetanide prescribing information recommends against concomitant use of probenecid. Monitor therapy
LORazepam: Probenecid may increase the serum concentration of LORazepam. Consider therapy modification
Lumateperone: Probenecid may increase the serum concentration of Lumateperone. Avoid combination
Meropenem: Probenecid may increase the serum concentration of Meropenem. Avoid combination
Methotrexate: Probenecid may increase the serum concentration of Methotrexate. Management: Avoid concomitant use of probenecid and methotrexate if possible. If used together, consider lower methotrexate doses and monitor for evidence of methotrexate toxicity. Consider therapy modification
Minoxidil (Systemic): Probenecid may increase the serum concentration of Minoxidil (Systemic). Monitor therapy
Mycophenolate: Probenecid may increase the serum concentration of Mycophenolate. Monitor therapy
Nitrofurantoin: Probenecid may increase the serum concentration of Nitrofurantoin. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: Probenecid may increase the serum concentration of Nonsteroidal Anti-Inflammatory Agents. Monitor therapy
Oseltamivir: Probenecid may increase serum concentrations of the active metabolite(s) of Oseltamivir. Management: Consider a change in therapy when using oseltamivir together with probenecid; reduced oseltamivir dose may be necessary. Increase monitoring for adverse events, such as thrombocytopenia. Consider therapy modification
Pegloticase: Probenecid may enhance the adverse/toxic effect of Pegloticase. Specifically, Probenecid may blunt increases in serum urate that would signal an elevated risk of anaphylaxis and infusion reactions. Avoid combination
Penicillins: Probenecid may increase the serum concentration of Penicillins. Monitor therapy
Pexidartinib: UGT1A4 Inhibitors may increase the serum concentration of Pexidartinib. Management: Avoid use of UGT1A4 inhibitors and pexidartinib. If combined use is required, reduce the pexidartinib dose. If receving pexidartinib 800 mg or 600 mg daily, reduce to 200 mg twice daily. If receiving 400 mg/day, reduce to 200 mg/day. Consider therapy modification
PRALAtrexate: Probenecid may increase the serum concentration of PRALAtrexate. Monitor therapy
Propacetamol: Probenecid may increase serum concentrations of the active metabolite(s) of Propacetamol. Specifically, accetaminophen exposure may be increased. Probenecid may also limit the formation of at least one major non-toxic acetaminophen metabolite, possibly increasing the formation of the toxic NAPQI metabolite. Management: Consider limiting the use of propacetamide in patients who are also taking probenecid. Patients may be at an increased risk for toxicity, even if reduced propacetamide doses are used. Consider therapy modification
Quinolones: Probenecid may decrease the excretion of Quinolones. Specifically, probenecid may decreased the renal excretion of quinolone antibiotics. Probenecid may increase the serum concentration of Quinolones. Exceptions: Moxifloxacin (Systemic). Monitor therapy
Salicylates: May diminish the therapeutic effect of Probenecid. Monitor therapy
Sodium Benzoate: Probenecid may increase the serum concentration of Sodium Benzoate. Specifically, probenecid may inhibit the renal transport of the hippuric acid metabolite of sodium benzoate. Monitor therapy
Sodium Phenylacetate: Probenecid may increase the serum concentration of Sodium Phenylacetate. Specifically, probenecid may inhibit the renal transport of the phenylacetylglutamine metabolite of sodium phenylacetate. Monitor therapy
Sulfonylureas: Probenecid may decrease the protein binding of Sulfonylureas. Probenecid may increase the serum concentration of Sulfonylureas. Monitor therapy
Theophylline Derivatives: Probenecid may increase the serum concentration of Theophylline Derivatives. Exceptions: Aminophylline; Theophylline. Monitor therapy
Urea Cycle Disorder Agents: Probenecid may increase serum concentrations of the active metabolite(s) of Urea Cycle Disorder Agents. Specifically, concentrations of phenylacetate and phenylacetylglutamine may be increased. Monitor therapy
Zidovudine: Probenecid may decrease the metabolism of Zidovudine. Monitor therapy
Test Interactions
False-positive glucosuria with Clinitest®, a falsely high determination of theophylline has occurred and the renal excretion of phenolsulfonphthalein 17-ketosteroids and bromsulfophthalein (BSP) may be inhibited
Adverse Reactions
Frequency not defined.
Cardiovascular: Flushing
Central nervous system: Dizziness, headache, pain (costovertebral)
Dermatologic: Alopecia, dermatitis, pruritus, skin rash
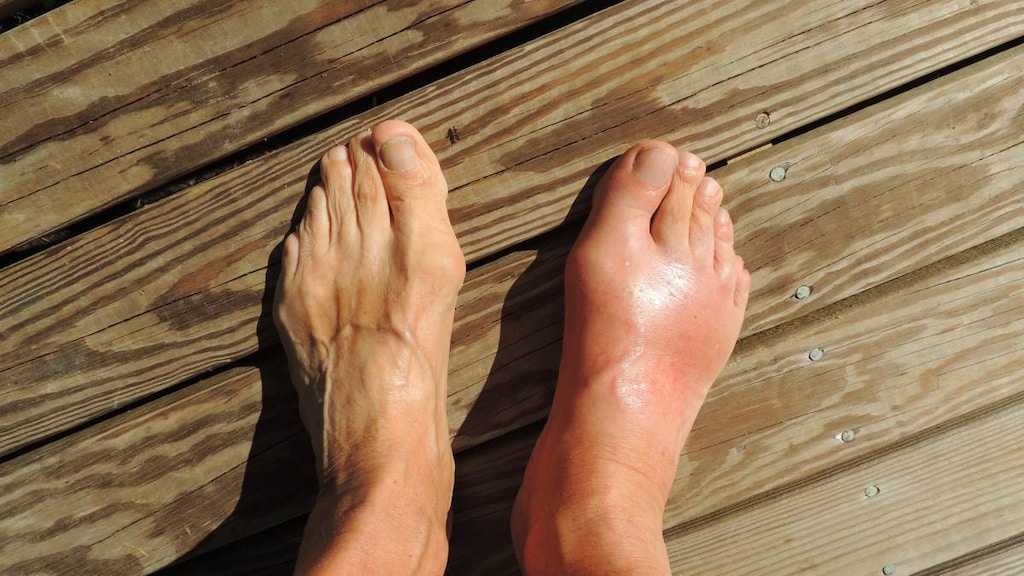
Endocrine & metabolic: Gouty arthritis (acute)
Gastrointestinal: Anorexia, dyspepsia, gastroesophageal reflux disease, gingival pain, nausea, vomiting
Genitourinary: Hematuria, nephrotic syndrome
Hematologic & oncologic: Anemia, aplastic anemia, hemolytic anemia (in G6PD deficiency), leukopenia
Hepatic: Hepatic necrosis
Hypersensitivity: Anaphylaxis, hypersensitivity reaction
Renal: Polyuria, renal colic
Miscellaneous: Fever
Warnings/Precautions
Concerns related to adverse effects:
- Allergic reaction: Has been associated with rare, severe hypersensitivity reactions, including anaphylaxis. Discontinue therapy if reaction occurs.
- Gout: May cause exacerbation of acute gouty attack.
Disease-related concerns:
- G6PD deficiency: Use caution in patients with G6PD deficiency; may increase risk for hemolytic anemia.
- Peptic ulcer disease: Use with caution in patients with peptic ulcer disease.
- Renal impairment: Monotherapy may not be effective in patients with a creatinine clearance <30 mL/minute. The American College of Rheumatology guidelines for the treatment of hyperuricemia do not recommend probenecid as first-line or an alternative first-line therapy in patients with a creatinine clearance <50 mL/minute (ACR guidelines [Khanna, 2012]).
Concurrent drug therapy issues:
- Methotrexate: Probenecid may increase the serum concentration of methotrexate. Avoid concomitant use of probenecid and methotrexate if possible. If used together, consider lower methotrexate doses and monitor for methotrexate toxicity.
- Penicillin: Use of probenecid with penicillin in patients with renal insufficiency is not recommended.
- Salicylates: Salicylates may diminish the therapeutic effect of probenecid; this effect may be more pronounced with high, chronic doses, however, the manufacturer recommends the use of an alternative analgesic even in place of small doses of aspirin.
Monitoring Parameters
Serum uric acid, urinary uric acid, renal function, CBC, frequency of gout attacks (Khanna 2012)
Pregnancy
Pregnancy Considerations
Probenecid crosses the placenta. Based on available data, an increased risk of adverse fetal events have not been reported (Gutman, 2012).
Patient Education
What is this drug used for?
- It is used to lower uric acid in the blood.
- It is used with some antibiotics to help fight the infection.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Dizziness
- Headache
- Lack of appetite
- Flushing
- Nausea
- Vomiting
- Gum swelling
- Gum soreness
- Hair loss
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Kidney problems like unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain.
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Kidney stone like back pain, abdominal pain, or blood in the urine.
- Sore throat
- Chills
- Bruising
- Bleeding
- Severe loss of strength and energy
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.