What is Spiriva Handihaler?
- Spiriva Handihaler is a prescription medicine used each day (a maintenance medicine) to control symptoms of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema.
- Spiriva Handihaler helps make your lungs work better for 24 hours. Spiriva Handihaler relaxes your airways and helps keep them open. You may start to feel like it is easier to breathe on the first day, but it may take longer for you to feel the full effects of the medicine. Spiriva Handihaler works best and may help make it easier to breathe when you use it every day.
- Spiriva Handihaler reduces the likelihood of flare-ups and worsening of COPD symptoms (COPD exacerbations). A COPD exacerbation is defined as an increase or new onset of more than one COPD symptom such as cough, mucus, shortness of breath, and wheezing that requires medicine beyond your rescue medicine.
Spiriva Handihaler is not a rescue medicine and should not be used for treating sudden breathing problems. Your doctor may give you other medicine to use for sudden breathing problems.
It is not known if Spiriva Handihaler is safe and effective in children.
What is COPD (Chronic Obstructive Pulmonary Disease)?
COPD is a serious lung disease that includes chronic bronchitis, emphysema, or both. Most COPD is caused by smoking. When you have COPD, your airways become narrow. So, air moves out of your lungs more slowly. This makes it hard to breathe.
What is the most important information I should know about Spiriva Handihaler?
Do not swallow Spiriva capsules. Spiriva capsules should only be used with the Handihaler device and inhaled through your mouth (oral inhalation).
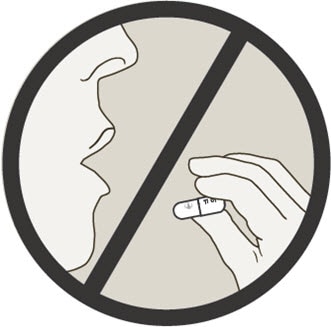
Who should not take Spiriva Handihaler?
Do not use Spiriva Handihaler if you:
- are allergic to tiotropium, ipratropium (Atrovent), or any of the ingredients in Spiriva Handihaler. See below for a complete list of ingredients in Spiriva Handihaler.
Symptoms of a serious allergic reaction to Spiriva Handihaler may include:
- raised red patches on your skin (hives)
- itching
- rash
- swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing
If you have these symptoms of an allergic reaction, stop taking Spiriva Handihaler and call your doctor right away or go to the nearest hospital emergency room.
What should I tell my healthcare provider before taking Spiriva Handihaler?
Before taking Spiriva Handihaler, tell your doctor about all your medical conditions, including if you:
- have kidney problems.
- have glaucoma. Spiriva Handihaler may make your glaucoma worse.
- have an enlarged prostate, problems passing urine, or a blockage in your bladder. Spiriva Handihaler may make these problems worse.
- are pregnant or plan to become pregnant. It is not known if Spiriva Handihaler could harm your unborn baby.
- are breast-feeding or plan to breast-feed. It is not known if Spiriva Handihaler passes into breast milk. You and your doctor will decide if Spiriva Handihaler is right for you while you breast-feed.
- have a severe allergy to milk proteins. Ask your doctor if you are not sure.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines and eye drops, vitamins, and herbal supplements. Some of your other medicines or supplements may affect the way Spiriva Handihaler works. Spiriva Handihaler is an anticholinergic medicine. You should not take other anticholinergic medicines while using Spiriva Handihaler, including ipratropium. Ask your doctor or pharmacist if you are not sure if one of your medicines is an anticholinergic.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist when you get a new medicine.
How should I take Spiriva Handihaler?
- Use Spiriva Handihaler exactly as prescribed. Use Spiriva Handihaler one time every day.
- Read the "Instructions for Use" that came with your medication before you use Spiriva Handihaler. Talk with your doctor if you do not understand the instructions.
- Do not swallow Spiriva capsules.
- Only use Spiriva capsules with the Handihaler device.
- Do not use the Handihaler device to take any other medicine.
- Spiriva Handihaler comes as a powder in a Spiriva capsule that fits the Handihaler device. Each Spiriva capsule, containing only a small amount of Spiriva powder, is one full dose of medicine.
- Separate one blister from the blister card. Then take out one of the Spiriva capsules from the blister package right before you use it.
- After the capsule is pierced, take a complete dose of Spiriva Handihaler by breathing in the powder by mouth two times, using the Handihaler device (take 2 inhalations from one Spiriva capsule). See the "instructions for use".
- Throw away any Spiriva capsule that is not used right away after it is taken out of the blister package. Do not leave the Spiriva capsules open to air; they may not work as well.
- If you miss a dose, take it as soon as you remember. Do not use Spiriva Handihaler more than one time every 24 hours.
- If you use more than your prescribed dose of Spiriva Handihaler, call your doctor or a poison control center.
What should I avoid while using Spiriva Handihaler?
- Do not let the powder from the Spiriva capsule get into your eyes. Your vision may get blurry and the pupil in your eye may get larger (dilate). If this happens, call your doctor.
- Spiriva Handihaler can cause dizziness and blurred vision. Should you experience these symptoms you should use caution when engaging in activities such as driving a car or operating appliances or other machines.
What are the possible side effects of Spiriva Handihaler?
Spiriva Handihaler can cause serious side effects, including:
- Allergic reaction. Symptoms may include:
- raised red patches on your skin (hives)
- itching
- rash
- swelling of the lips, tongue, or throat that may cause difficulty in breathing or swallowing
If you have these symptoms of an allergic reaction, stop taking Spiriva Handihaler and call your doctor right away or go to the nearest hospital emergency room.
- Sudden narrowing and blockage of the airways into the lungs (bronchospasm). Your breathing suddenly gets worse.
If you have these symptoms of bronchospasm, stop taking Spiriva Handihaler and call your doctor right away or go to the nearest hospital emergency room.
- New or worsened increased pressure in the eyes (acute narrow-angle glaucoma). Symptoms of acute narrow-angle glaucoma may include:
- eye pain
- blurred vision
- seeing halos (visual halos) or colored images along with red eyes
Using only eye drops to treat these symptoms may not work. If you have these symptoms, stop taking Spiriva Handihaler and call your doctor right away.
- New or worsened urinary retention. Symptoms of blockage in your bladder and/or enlarged prostate may include:
- difficulty passing urine,
- painful urination.
If you have these symptoms of urinary retention, stop taking Spiriva Handihaler and call your doctor right away.
Other side effects with Spiriva Handihaler include:
- upper respiratory tract infection
- dry mouth
- sinus infection
- sore throat
- non-specific chest pain
- urinary tract infection
- indigestion
- runny nose
- constipation
- increased heart rate
- blurred vision
These are not all the possible side effects with Spiriva Handihaler. Tell your doctor if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Spiriva Handihaler Images
Drug Interactions
A total of 111 medications are known to interact with Spiriva Handihaler. Use the Interactions Checker Tool.
Common Interactions Checks
General information about the safe and effective use Spiriva Handihaler
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use Spiriva Handihaler for a purpose for which it has not been prescribed. Do not give Spiriva Handihaler to other people even if they have the same symptoms that you have. It may harm them.
For more information about Spiriva Handihaler, talk with your doctor. You can ask your doctor or pharmacist for information about Spiriva Handihaler that is written for health professionals.
For more information about Spiriva Handihaler, go to Spiriva Handihaler information, or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257 or (TTY) 1-800-459-9906.
How do I store Spiriva Handihaler?
- Do not store Spiriva capsules in the Handihaler device.
- Store Spiriva capsules in the sealed blister package at room temperature 68°F to 77°F (20°C to 25°C).
- Keep Spiriva capsules away from heat and cold (do not freeze).
- Store Spiriva capsules in a dry place. Throw away any unused Spiriva capsules that have been open to air.
Ask your doctor or pharmacist if you have any questions about storing your Spiriva capsules.
Keep Spiriva Handihaler, Spiriva capsules, and all medicines out of the reach of children.
What are the ingredients in Spiriva Handihaler?
Active ingredient: tiotropium
Inactive ingredient: lactose monohydrate





