Boxed Warning
Hematologic toxicity:
Zidovudine has been associated with hematologic toxicity, including neutropenia and severe anemia, particularly in patients with advanced HIV-1 disease.
Myopathy:
Prolonged use of zidovudine has been associated with symptomatic myopathy.
Lactic acidosis/severe hepatomegaly:
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination, including with zidovudine and other antiretrovirals. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Capsule, Oral:
Retrovir: 100 mg [contains soybean lecithin]
Generic: 100 mg
Solution, Intravenous [preservative free]:
Retrovir: 10 mg/mL (20 mL)
Syrup, Oral:
Retrovir: 50 mg/5 mL (240 mL) [contains sodium benzoate; strawberry flavor]
Generic: 50 mg/5 mL (240 mL)
Tablet, Oral:
Generic: 300 mg
Pharmacology
Mechanism of Action
Zidovudine is a thymidine analog which interferes with the HIV viral RNA-dependent DNA polymerase resulting in inhibition of viral replication; nucleoside reverse transcriptase inhibitor
Pharmacokinetics/Pharmacodynamics
Absorption
Oral: Well absorbed
Distribution
Significant penetration into the CSF
Vd: 1 to 2.2 L/kg
CSF/plasma ratio:
Infants 3 months to Children 12 years (n=38): Median: 0.68; Range: 0.03 to 3.25
Adults (n=39): Median: 0.6; Range: 0.04 to 2.62
Metabolism
Hepatic via glucuronidation to inactive metabolites, including GZDV; extensive first-pass effect
Excretion
Oral: Urine (72% to 74% as metabolites, 14% to 18% as unchanged drug)
IV: Urine (45% to 60% as metabolites, 18% to 29% as unchanged drug)
Time to Peak
Serum: 30 to 90 minutes
Half-Life Elimination
Terminal:
Premature neonate: 6.3 hours
Full-term neonates: 3.1 hours
Infants 14 days to 3 months: 1.9 hours
Infants 3 months to Children 12 years: 1.5 hours
Adults: 0.5 to 3 hours (mean 1.1 hours)
Protein Binding
25% to 38%
Use in Specific Populations
Special Populations: Renal Function Impairment
Cl is decreased, resulting in an increased half-life and AUC of zidovudine and major metabolite GZDV.
Special Populations: Hepatic Function Impairment
Clearance is decreased and plasma concentrations are increased.
Use: Labeled Indications
HIV-1 infection, treatment: Treatment of HIV-1 infection in combination with other antiretroviral agents
Perinatal HIV-1 transmission, prevention: Prevention of mother to fetus HIV-1 transmission
Use: Off Label
HIV-1 nonoccupational postexposure prophylaxis (nPEP)yes
Based on the Centers for Disease Control and Prevention (CDC), US Department of Health and Human Services updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV, zidovudine is an effective and recommended treatment option (in conjunction with other antiretrovirals) as postexposure prophylaxis of HIV-1 infection following nonoccupational exposure (nPEP) in individuals exposed to blood, genital secretions, or other potentially infectious body fluids that may contain HIV when that exposure represents a substantial risk for HIV transmission.
Contraindications
Potentially life-threatening hypersensitivity to zidovudine or any component of the formulation
Canadian labeling: Additional contraindications (not in US labeling): Neutrophil count <750/mm3 or hemoglobin <7.5 g/dL (4.65 mmol/L)
Dosage and Administration
Dosing: Adult
Note: Patients should receive IV therapy only until oral therapy can be administered.
Prevention of perinatal HIV transmission: Zidovudine should be administered by continuous IV infusion near delivery regardless of antepartum regimen or mode of delivery in women with known or suspected HIV RNA >1,000 copies/mL or unknown HIV RNA status; use may be considered in women with HIV RNA between 50 and 999 copies/mL. If oral zidovudine was part of the antepartum regimen, discontinue during intrapartum IV infusion. Other antiretroviral agents should be continued orally. Zidovudine IV is not required in women receiving combination antiretroviral therapy who have HIV RNA <50 copies/mL near delivery and there are no concerns related to adherence with the regimen (HHS [perinatal] 2019).
IV (preferred route): During labor and delivery: Loading dose: 2 mg/kg followed by a continuous IV infusion of 1 mg/kg/hour until clamping of the umbilical cord. For scheduled cesarean delivery, begin IV zidovudine 3 hours before surgery. In cases of unscheduled cesarean delivery due to maternal and fetal indications, consider administering the loading dose then proceeding to delivery. Note: Dosage based on total body weight.
Oral (if IV not possible): Loading dose: 600 mg, then 400 mg every 3 hours (oral is not the preferred route of administration in the United States) (HHS [perinatal] 2019)
HIV-1 infection, treatment: Note: Use in combination with other antiretroviral agents.
Oral: 300 mg twice daily
IV: 1 mg/kg/dose administered every 4 hours around-the-clock (6 doses daily)
HIV-1 nonoccupational postexposure prophylaxis (nPEP) (off-label use) (HHS [nPEP] 2016): Oral:
CrCl ≥60 mL/minute: Zidovudine is not a component of the recommended antiretroviral regimen for these patients.
CrCl <60 and ≥15 mL/minute: 300 mg twice daily in combination with other antiretroviral agents. Initiate therapy within 72 hours of exposure and continue for 28 days
CrCl <15 mL/minute: Adjust dose based on renal function. Give in combination with other antiretroviral agents. Initiate therapy within 72 hours of exposure and continue for 28 days
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
HIV-1 infection, treatment: Use in combination with other antiretroviral (ARV) agents. Gene mutation and ARV resistance patterns should be evaluated (refer to https://www.iasusa.org for more information) when necessary.
Oral: Infants (born at or near term ≥35 weeks postconception, and PNA ≥4 weeks) weighing ≥4 kg, Children, and Adolescents: Note: The 3-times-daily dosing is FDA approved but rarely used in practice; twice daily dosing is recommended in the current pediatric HIV guidelines (HHS [pediatric] 2018).
Weight-directed dosing:
4 to <9 kg: 12 mg/kg/dose twice daily or 8 mg/kg/dose 3 times daily.
9 to <30 kg: 9 mg/kg/dose twice daily or 6 mg/kg/dose 3 times daily.
≥30 kg: 300 mg twice daily or 200 mg 3 times daily.
BSA-directed dosing: 240 mg/m2/dose every 12 hours, range: 180 to 240 mg/m2/dose every 12 hours (maximum dose: 300 mg/dose) or 160 mg/m2/dose every 8 hours (maximum dose: 200 mg/dose).
IV: Note: Use IV route only until oral therapy can be administered:
Infants ≥3 months and Children: 120 mg/m2/dose every 6 hours; maximum dose: 160 mg/dose (Retrovir prescribing information [Canada] 2018).
Adolescents ≥30 kg: 1 to 2 mg/kg every 4 hours around the clock (Retrovir prescribing information [Canada] 2018).
HIV-1 perinatal transmission, prevention (HHS [perinatal] 2018): Note: Dosing addresses completion of courses initiated at birth; if the neonate is diagnosed as HIV positive, discontinue empiric dosing and transition to a treatment regimen and monitoring (see HIV-1 infection, treatment). Duration of zidovudine in general is 6 weeks, although is variable, and addition/choice of additional antiretrovirals (eg, lamivudine, raltegravir) depends on risk of transmission, serology testing, and chosen strategy; see guidelines for additional information on high-risk definitions (HHS [perinatal] 2018).
Infants born prematurely (GA <35 weeks): Refer to Dosing: Neonatal; standard infant doses may be excessive in infants who were born prematurely.
Infants <6 weeks (GA ≥35 weeks) (HHS [perinatal] 2018):
Oral:
Weight-directed dosing: 4 mg/kg/dose every 12 hours.
Fixed weight-band dosing regimen:
2 to <3 kg: 10 mg every 12 hours.
3 to <4 kg: 15 mg every 12 hours.
4 to <5 kg: 20 mg every 12 hours.
IV: 3 mg/kg/dose every 12 hours. Note: Use IV route only until oral therapy can be administered.
HIV-1 nonoccupational postexposure prophylaxis (nPEP) (HHS [nPEP] 2016): Note: Initiate therapy within 72 hours of exposure and continue for 28 days; use in combination with other antiretroviral agents.
Infants (PCA ≥35 weeks and PNA ≥4 weeks of age) and Children: Oral:
4 to <9 kg: 12 mg/kg/dose twice daily.
9 to <30 kg: 9 mg/kg/dose twice daily.
≥30 kg: 300 mg twice daily.
Adolescents: Oral: 300 mg twice daily.
Dosing adjustment for hematologic toxicity: Consider interruption of therapy for significant anemia (Hgb <7.5 g/dL or >25% decrease from baseline) and/or significant neutropenia (ANC <750 cells/mm3 or >50% decrease from baseline) until evidence of bone marrow recovery occurs; once bone marrow recovers, dose may be resumed using appropriate adjunctive therapy (eg, epoetin alfa).
Dosing: Adjustment for Toxicity
Consider dose interruption for significant anemia (hemoglobin <7.5 g/dL or >25% reduction from baseline) and/or neutropenia (granulocyte count <750 cells/mm3 or >50% reduction from baseline) until evidence of recovery. Resumption in dose in combination with adjunctive measures (eg, epoetin alfa) may be appropriate, depending on erythropoietin level and patient tolerance.
Reconstitution
Solution for injection should be removed from the vial and diluted with D5W to a concentration ≤4 mg/mL.
Administration
Oral: Administer without regard to meals.
IV: Avoid rapid infusion or bolus injection. Do not administer IM.
Infuse over 1 hour; in pregnant women, infuse loading dose over 1 hour followed by continuous infusion.
Storage
IV: Store undiluted vials at 15°C to 25°C (59°F to 77°F). Protect from light. When diluted in D5W, solution is physically and chemically stable for 24 hours at room temperature and 48 hours if refrigerated at 2°C to 8°C (36°F to 46°F). Attempt to administer diluted solution within 8 hours if stored at room temperature or 24 hours if refrigerated at 2°C to 8°C (36°F to 46°F) to minimize potential for microbial-contaminated solutions (vials are single-use and do not contain preservative).
Tablets, capsules, syrup: Store at 15°C to 25°C (59°F to 77°F). Protect capsules from moisture.
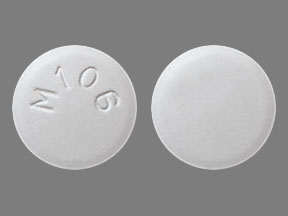
Zidovudine Images
Drug Interactions
Acemetacin: May enhance the adverse/toxic effect of Zidovudine. Specifically, the risk for hematologic toxicity may be increased. Monitor therapy
Acyclovir-Valacyclovir: May enhance the CNS depressant effect of Zidovudine. Monitor therapy
Amodiaquine: Zidovudine may enhance the neutropenic effect of Amodiaquine. Avoid combination
BCG (Intravesical): Myelosuppressive Agents may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Chloramphenicol (Ophthalmic): May enhance the adverse/toxic effect of Myelosuppressive Agents. Monitor therapy
Cladribine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Avoid combination
Cladribine: Agents that Undergo Intracellular Phosphorylation may diminish the therapeutic effect of Cladribine. Avoid combination
Clarithromycin: May enhance the myelosuppressive effect of Zidovudine. Clarithromycin may decrease the serum concentration of Zidovudine. Management: Monitor response to zidovudine closely when used with clarithromycin, and consider staggering zidovudine and clarithromycin doses when possible in order to minimize the potential for interaction. Consider therapy modification
CloZAPine: Myelosuppressive Agents may enhance the adverse/toxic effect of CloZAPine. Specifically, the risk for neutropenia may be increased. Monitor therapy
Deferiprone: Myelosuppressive Agents may enhance the neutropenic effect of Deferiprone. Management: Avoid the concomitant use of deferiprone and myelosuppressive agents whenever possible. If this combination cannot be avoided, monitor the absolute neutrophil count more closely. Consider therapy modification
Dexketoprofen: May enhance the adverse/toxic effect of Zidovudine. Monitor therapy
Dipyrone: May enhance the adverse/toxic effect of Myelosuppressive Agents. Specifically, the risk for agranulocytosis and pancytopenia may be increased Avoid combination
DOXOrubicin (Conventional): May enhance the adverse/toxic effect of Zidovudine. DOXOrubicin (Conventional) may diminish the therapeutic effect of Zidovudine. Consider therapy modification
DOXOrubicin (Liposomal): May enhance the adverse/toxic effect of Zidovudine. DOXOrubicin (Liposomal) may diminish the therapeutic effect of Zidovudine. Consider therapy modification
Fluconazole: May decrease the metabolism of Zidovudine. Monitor therapy
Ganciclovir-Valganciclovir: May enhance the adverse/toxic effect of Zidovudine. Specifically, hematologic toxicity may be enhanced. Monitor therapy
Interferons: May enhance the adverse/toxic effect of Zidovudine. Interferons may decrease the metabolism of Zidovudine. Monitor therapy
Levomethadone: May increase the serum concentration of Zidovudine. Monitor therapy
Mesalamine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Methadone: May increase the serum concentration of Zidovudine. Monitor therapy
Nitisinone: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Orlistat: May decrease the serum concentration of Antiretroviral Agents. Monitor therapy
Pretomanid: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Probenecid: May decrease the metabolism of Zidovudine. Monitor therapy
Promazine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Protease Inhibitors: May decrease the serum concentration of Zidovudine. Monitor therapy
Raltegravir: May enhance the myopathic (rhabdomyolysis) effect of Zidovudine. Monitor therapy
Ribavirin (Oral Inhalation): Zidovudine may enhance the adverse/toxic effect of Ribavirin (Oral Inhalation). Specifically, the risk/severity of anemia may be increased. Management: Due to significantly increased risk of anemia, consider even closer monitoring for anemia than routinely recommended. Alternative therapies should be considered when clinically possible, particularly for patients with other risk factors. Consider therapy modification
Ribavirin (Systemic): Zidovudine may enhance the adverse/toxic effect of Ribavirin (Systemic). Specifically, the risk/severity of anemia may be increased. Management: Due to significantly increased risk of anemia, consider even closer monitoring for anemia than routinely recommended for ribavirin. Alternative therapies should be considered when clinically possible, particularly for patients with other risk factors. Consider therapy modification
Rifamycin Derivatives: May decrease the serum concentration of Zidovudine. Exceptions: Rifabutin. Monitor therapy
Stavudine: Zidovudine may diminish the therapeutic effect of Stavudine. Avoid combination
Tenoxicam: May enhance the adverse/toxic effect of Zidovudine. Monitor therapy
Teriflunomide: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Tolvaptan: May increase the serum concentration of OAT1/3 Substrates. Management: Patients being treated with the Jynarque brand of tolvaptan should avoid concomitant use of OAT1/3 substrates. Concentrations and effects of the OAT1/3 substrate would be expected to increase with any combined use. Consider therapy modification
Valproate Products: May increase the serum concentration of Zidovudine. Monitor therapy
Adverse Reactions
Percentages noted with oral administration in adults unless otherwise stated. Pediatric adverse event incidences occurred with combination therapy.
>10%:
Central nervous system: Headache (63%), malaise (53%)
Dermatologic: Skin rash (infants, children, & adolescents: 12%)
Gastrointestinal: Nausea (adults: 51%; infants, children, & adolescents: 8%), anorexia (20%), vomiting (adults: 17%; infants, children, & adolescents: 8%)
Hematologic & oncologic: Macrocytosis (infants, children, & adolescents: >50%), anemia (neonates: 22%; infants, children, & adolescents: 4%; adults, grades 3/4: 1%)
Hepatic: Hepatomegaly (infants, children, & adolescents: 11%)
Respiratory: Cough (infants, children, & adolescents: 15%)
Miscellaneous: Fever (infants, children, & adolescents: 25%)
1% to 10%:
Cardiovascular: Cardiac failure (infants, children, & adolescents: <6%), ECG abnormality (infants, children, & adolescents: <6%), edema (infants, children, & adolescents: <6%), left ventricular dilation (infants, children, & adolescents: <6%)
Central nervous system: Hyporeflexia (infants, children, & adolescents: <6%), irritability (infants, children, & adolescents: <6%), nervousness (infants, children, & adolescents: <6%), chills (≥5%), fatigue (≥5%), insomnia (≥5%), neuropathy (≥5%)
Endocrine & metabolic: Weight loss (infants, children, & adolescents: <6%), increased amylase (infants, children, & adolescents, grades 3/4: 3%)
Gastrointestinal: Diarrhea (infants, children, & adolescents: 8%), constipation (6%), stomatitis (infants, children, & adolescents: 6%), abdominal cramps (≥5%), abdominal pain (≥5%), dyspepsia (≥5%), increased serum lipase (infants, children, & adolescents, grades 3/4: 3%)
Genitourinary: Hematuria (infants, children, & adolescents: <6%)
Hematologic & oncologic: Lymphadenopathy (infants, children, & adolescents: 9%), neutropenia (infants, children, & adolescents, grades 3/4: 8%), splenomegaly (infants, children, & adolescents: 5%), thrombocytopenia (infants, children, & adolescents, grades 3/4: 1%)
Hepatic: Increased serum aspartate aminotransferase (infants, children, & adolescents, grades 3/4: 2%), increased serum alanine aminotransferase (infants, children, & adolescents, grades 3/4: 1%),
Neuromuscular & skeletal: Asthenia (9%), arthralgia (≥5%), musculoskeletal pain (≥5%), myalgia (≥5%)
Otic: Ear sign or symptom (infants, children, & adolescents: 7%)
Respiratory: Nasal congestion (infants, children, & adolescents: ≤8%), rhinorrhea (infants, children, & adolescents: ≤8%), abnormal breath sounds (infants, children, & adolescents: ≤7%), wheezing (infants, children, & adolescents: ≤7%)
Frequency not defined:
Local: Injection site reaction (IV), irritation at injection site (IV), pain at injection site (IV)
<1%, postmarketing and/or case reports: Amblyopia, anaphylaxis, angioedema, anxiety, aplastic anemia, autoimmune disease, back pain, cardiomyopathy, chest pain, confusion, decreased mental acuity, depression, diaphoresis, dizziness, drowsiness, dyschromia, dysgeusia, dysphagia, dyspnea, flatulence, flu-like symptoms, Graves disease, Guillain-Barré syndrome, gynecomastia, hearing loss, hemolytic anemia, hepatitis, hepatomegaly with steatosis, hyperbilirubinemia, hypersensitivity reaction, immune reconstitution syndrome, increased creatine phosphokinase, increased lactate dehydrogenase, jaundice, lactic acidosis, leukopenia, lipotrophy, macular edema, mania, muscle spasm, myopathy, myositis, oral mucosa hyperpigmentation, oral mucosa ulcer, pain, pancreatitis, pancytopenia, paresthesia, photophobia, polymyositis, pruritus, pure red cell aplasia, redistribution of body fat, rhabdomyolysis, rhinitis, seizure, sinusitis, Stevens-Johnson syndrome, syncope, toxic epidermal necrolysis, tremor, urinary frequency, urinary hesitancy, urticaria, vasculitis, vertigo
Warnings/Precautions
Concerns related to adverse effects:
- Hematologic toxicity: [US Boxed Warning]: Hematologic toxicity, including neutropenia and severe anemia have been reported with use, especially with advanced HIV-1 disease. Toxicity may be related to duration of use and prior bone marrow reserve. Hemoglobin reduction may occur in as early as 2 to 4 weeks; neutropenia usually occurs after 6 to 8 weeks. Pancytopenia has been reported (usually reversible). Use with caution in patients with bone marrow compromise (granulocytes <1,000 cells/mm3 or hemoglobin <9.5 g/dL). Monitor blood counts; dose interruption may be required in patients who develop anemia or neutropenia.
- Immune reconstitution syndrome: Patients may develop immune reconstitution syndrome resulting in the occurrence of an inflammatory response to an indolent or residual opportunistic infection during initial HIV treatment or activation of autoimmune disorders (eg, Graves disease, polymyositis, Guillain-Barré syndrome) later in therapy; further evaluation and treatment may be required.
- Lactic acidosis/hepatomegaly: [US Boxed Warning]: Lactic acidosis and severe hepatomegaly with steatosis have been reported with nucleoside analogues, including fatal cases. Risk may be increased with obesity or in females. Suspend treatment in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or hepatotoxicity (transaminase elevation may/may not accompany hepatomegaly and steatosis).
- Lipoatrophy: May cause loss of subcutaneous fat, especially in the face, limbs, and buttocks. Lipoatrophy incidence and severity are related to cumulative exposure and may be only partially reversible; improvement may take months to years after switching to a regimen that does not contain zidovudine. Monitor patients for signs of lipoatrophy and consider switching to a non-zidovudine-containing regimen if lipoatrophy occurs.
- Myopathy: [US Boxed Warning]: Prolonged use has been associated with symptomatic myopathy and myositis. Pathological changes observed are similar to that produced by HIV-1 disease.
Disease-related concerns:
- Hepatic impairment: Hematologic toxicity may be increased due to increased serum concentrations in patients with hepatic impairment.
- Renal impairment: Use with caution in patients with CrCl <15 mL/minute; dosage adjustment recommended.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Newborns: Zidovudine newborn prophylaxis may affect diagnostic virologic assays in HIV-exposed infants. If a virologic assay result is negative while the infant is receiving combination antiretroviral prophylaxis, repeat virologic testing should be considered 2 to 4 weeks after cessation of antiretroviral prophylaxis (HHS [pediatric] 2018).
Dosage form specific issues:
- Benzyl alcohol and derivatives: Some dosage forms may contain sodium benzoate/benzoic acid; benzoic acid (benzoate) is a metabolite of benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC, 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol derivative with caution in neonates. See manufacturer's labeling.
Dosage form specific issues:
- Injection: Latex is used in vial stopper. May cause allergic reactions in latex-sensitive individuals.
Monitoring Parameters
CBC with differential (more frequent monitoring required in patients with poor bone marrow reserve); LFTs; serum creatinine; HIV viral load and CD4 count.
Pregnancy
Pregnancy Considerations
Zidovudine has a high level of transfer across the human placenta; the placenta also metabolizes zidovudine to the active metabolite.
No increased risk of overall birth defects has been observed following first trimester exposure according to data collected by the antiretroviral pregnancy registry. Maternal antiretroviral therapy (ART) may be associated with adverse pregnancy outcomes including preterm delivery, stillbirth, low birth weight, and small for gestational age infants. Actual risks may be influenced by maternal factors, such as disease severity, gestational age at initiation of therapy, and specific ART regimen, therefore close fetal monitoring is recommended. Because there is clear benefit to appropriate treatment, maternal ART should not be withheld due to concerns for adverse neonatal outcomes. Long-term follow-up is recommended for all infants exposed to antiretroviral medications; children without HIV but who were exposed to ART in utero and develop significant organ system abnormalities of unknown etiology (particularly of the CNS or heart) should be evaluated for potential mitochondrial dysfunction. Cases of lactic acidosis and hepatic steatosis have been reported in pregnant women with use of nucleoside reverse transcriptase inhibitors (NRTIs).
The Health and Human Services (HHS) perinatal HIV guidelines consider zidovudine an alternative NRTI for pregnant females living with HIV who are antiretroviral-naive, who have had ART therapy in the past but are restarting, who require a new ART regimen (due to poor tolerance or poor virologic response of current regimen), and who are not yet pregnant but are trying to conceive. In addition, females who become pregnant while taking zidovudine may continue if viral suppression is effective and the regimen is well tolerated. The pharmacokinetics of zidovudine are not significantly altered in pregnancy and dosing adjustment is not needed.
The HHS perinatal HIV guidelines consider zidovudine in combination with lamivudine as an alternative NRTI backbone for initial therapy in antiretroviral-naive pregnant females. Zidovudine should be administered IV near delivery regardless of antepartum regimen or mode of delivery in females with known or suspected HIV RNA >1,000 copies/mL or unknown HIV RNA status (even in cases of documented zidovudine resistance) and may be considered in females with HIV RNA between 50 to 999 copies/mL, unless there is a history of hypersensitivity.
In general, ART is recommended for all pregnant females living with HIV to keep the viral load below the limit of detection and reduce the risk of perinatal transmission. Therapy should be individualized following a discussion of the potential risks and benefits of treatment during pregnancy. Monitoring of pregnant females is more frequent than in nonpregnant adults. ART should be continued postpartum for all females living with HIV and can be modified after delivery.
Health care providers are encouraged to enroll pregnant females exposed to antiretroviral medications as early in pregnancy as possible in the Antiretroviral Pregnancy Registry (1-800-258-4263 or http://www.APRegistry.com). Health care providers caring for pregnant females living with HIV and their infants may contact the National Perinatal HIV Hotline (1-888-448-8765) for clinical consultation (HHS [perinatal] 2019).
Patient Education
What is this drug used for?
- It is used to treat HIV infection.
- It is used to keep the HIV infection from passing from the mother to the newborn during delivery. The newborn will also be given this drug after birth.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Constipation
- Headache
- Lack of appetite
- Vomiting
- Nausea
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Infection
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Lactic acidosis like fast breathing, fast heartbeat, abnormal heartbeat, vomiting, fatigue, shortness of breath, severe loss of strength and energy, severe dizziness, feeling cold, or muscle pain or cramps.
- Pancreatitis like severe abdominal pain, severe back pain, severe nausea, or vomiting.
- Severe loss of strength and energy
- Muscle pain
- Muscle weakness
- Bruising
- Bleeding
- Change in body fat
- Stevens-Johnson syndrome/toxic epidermal necrolysis like red, swollen, blistered, or peeling skin (with or without fever); red or irritated eyes; or sores in mouth, throat, nose, or eyes.
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.