What is Zyprexa Relprevv?
Zyprexa Relprevv is a long-acting prescription medicine given by injection and used to treat schizophrenia in adults. The symptoms of schizophrenia include:
- hearing voices
- seeing things that are not there
- having beliefs that are not true
- being suspicious or withdrawn
Some of your symptoms of schizophrenia may improve with treatment with Zyprexa Relprevv. If you do not think you are getting better, call your doctor.
It is not known if Zyprexa Relprevv is safe and effective in children under 18 years of age.
What is the most important information I should know about Zyprexa Relprevv?
Before you receive Zyprexa Relprevv treatment you must:
- understand the risks and benefits of Zyprexa Relprevv treatment. Your doctor will talk to you about the risks and benefits of Zyprexa Relprevv treatment.
- register in the Zyprexa Relprevv Patient Care Program. You must agree to the rules of the Zyprexa Relprevv Patient Care Program before you register.
Zyprexa Relprevv may cause serious side effects, including:
- Post-injection Delirium Sedation Syndrome (PDSS).
- Increased risk of death in elderly people who are confused, have memory loss and have lost touch with reality (dementia-related psychosis).
- High blood sugar (hyperglycemia).
- High fat levels in your blood (increased cholesterol and triglycerides), especially in teenagers age 13 to 17.
- Weight gain, especially in teenagers age 13 to 17.
These serious side effects are described below.
1. Post-injection Delirium Sedation Syndrome (PDSS). PDSS is a serious problem that can happen after you get a Zyprexa Relprevv injection if the medicine gets in your blood too fast. This problem usually happens within 3 hours after you receive Zyprexa Relprevv. If the medicine gets in your blood too fast, you may have some of the following symptoms:
- feel more sleepy than usual
- feel dizzy
- feel confused or disoriented
- trouble talking or walking
- muscles feel stiff or shaking
- feel weak
- feel grouchy or angry
- feel nervous or anxious
- higher blood pressure
- seizures (convulsions)
- pass out (become unconscious or coma)
You will need to stay at the clinic where you receive the injection for at least 3 hours so your doctor can make sure you do not have symptoms of PDSS. When you leave the clinic someone must be with you. If you have symptoms of PDSS after you leave the clinic, get medical help or go to an emergency room right away.
2. Increased risk of death in elderly people who are confused, have memory loss and have lost touch with reality (dementia-related psychosis). Zyprexa Relprevv is not approved for treating psychosis in elderly people with dementia.
3. High blood sugar (hyperglycemia). High blood sugar can happen if you have diabetes already or if you have never had diabetes. High blood sugar could lead to:
- a build up of acid in your blood due to ketones (ketoacidosis)
- coma
- death
Your doctor should do tests to check your blood sugar before you start taking Zyprexa Relprevv and during treatment. In people who do not have diabetes, sometimes high blood sugar goes away when Zyprexa Relprevv is stopped. People with diabetes and some people who did not have diabetes before taking Zyprexa Relprevv need to take medicine for high blood sugar even after they stop taking Zyprexa Relprevv.
If you have diabetes, follow your doctor's instructions about how often to check your blood sugar while taking Zyprexa Relprevv.
Call your doctor if you have any of these symptoms of high blood sugar (hyperglycemia) while taking Zyprexa Relprevv:
- feel very thirsty
- need to urinate more than usual
- feel very hungry
- feel weak or tired
- feel sick to your stomach
- feel confused or your breath smells fruity
4. High fat levels in your blood (cholesterol and triglycerides). High fat levels may happen in people treated with Zyprexa Relprevv, especially in teenagers (13 to 17 years old). Zyprexa Relprevv is not approved in patients less than 18 years old. You may not have any symptoms, so your doctor should do blood tests to check your cholesterol and triglyceride levels before you start taking Zyprexa Relprevv and during treatment.
5. Weight gain. Weight gain is very common in people who take Zyprexa Relprevv. Teenagers (13 to 17 years old) are more likely to gain weight and to gain more weight than adults. Zyprexa Relprevv is not approved in patients less than 18 years old. Some people may gain a lot of weight while taking Zyprexa Relprevv, so you and your doctor should check your weight regularly. Talk to your doctor about ways to control weight gain, such as eating a healthy, balanced diet, and exercising.
What should I tell my healthcare provider before taking Zyprexa Relprevv?
Zyprexa Relprevv may not be right for you. Before starting Zyprexa Relprevv, tell your doctor if you have or had:
- heart problems
- seizures
- diabetes or high blood sugar levels (hyperglycemia)
- high cholesterol or triglyceride levels in your blood
- liver problems
- low or high blood pressure
- strokes or “mini-strokes” also called transient ischemic attacks (TIAs)
- Alzheimer's disease
- narrow-angle glaucoma
- enlarged prostate in men
- bowel obstruction
- breast cancer
- thoughts of suicide or hurting yourself
- any other medical condition
- are pregnant or plan to become pregnant. It is not known if Zyprexa Relprevv will harm your unborn baby.
- If you become pregnant while receiving Zyprexa, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
- are breast-feeding or plan to breast-feed. Zyprexa Relprevv passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take Zyprexa Relprevv.
Tell your doctor if you exercise a lot or are in hot places often.
The symptoms of schizophrenia may include thoughts of suicide or of hurting yourself or others. If you have these thoughts at any time, tell your doctor or go to an emergency room right away.
Tell your doctor about all the medicines that you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Zyprexa Relprevv and some medicines may interact with each other and may not work as well, or cause possible serious side effects. Your doctor can tell you if it is safe to take Zyprexa Relprevv with your other medicines. Do not start or stop any medicine while taking Zyprexa Relprevv without talking to your doctor first.
How should I use Zyprexa Relprevv?
- Zyprexa Relprevv will be injected into the muscle in your buttock (gluteus) by your doctor or nurse at the clinic. See Instructions for use below.
- After receiving Zyprexa Relprevv you will need to stay at the clinic for at least 3 hours.
- When you leave the clinic, someone must be with you.
- Call your doctor if you do not think you are getting better or have any concerns about your condition while taking Zyprexa Relprevv.
What should I avoid while using Zyprexa Relprevv?
- Zyprexa Relprevv can cause sleepiness and may affect your ability to make decisions, think clearly, or react quickly. Do not drive, operate heavy machinery, or do other dangerous activities until you know how Zyprexa Relprevv affects you. You should not drive or operate heavy machinery for the rest of the day after each injection.
- Avoid drinking alcohol while taking Zyprexa Relprevv. Drinking alcohol while you take Zyprexa Relprevv may make you sleepier than if you take Zyprexa Relprevv alone.
What are the possible side effects of Zyprexa Relprevv?
Serious side effects may happen when you take Zyprexa Relprevv, including:
- See “What is the most important information I should know about Zyprexa Relprevv?”, which describes the risk of post-injection delirium sedation syndrome (PDSS), increased risk of death in elderly people with dementia-related psychosis and the risks of high blood sugar, high cholesterol and triglyceride levels, and weight gain.
- Increased incidence of stroke or “mini-strokes” called transient ischemic attacks (TIAs) in elderly people with dementia-related psychosis (elderly people who have lost touch with reality due to confusion and memory loss). Zyprexa Relprevv is not approved for these patients.
- Neuroleptic Malignant Syndrome (NMS): NMS is a rare but very serious condition that can happen in people who take antipsychotic medicines, including Zyprexa Relprevv. NMS can cause death and must be treated in a hospital. Call your doctor right away if you become severely ill and have any of these symptoms:
- high fever
- excessive sweating
- rigid muscles
- confusion
- changes in your breathing, heartbeat, and blood pressure
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): DRESS can occur with Zyprexa Relprevv. Features of DRESS may include rash, fever, swollen glands and other internal organ involvement such as liver, kidney, lung and heart. DRESS is sometimes fatal; therefore, tell your doctor immediately if you experience any of these signs.
- Tardive Dyskinesia: This condition causes body movements that keep happening and that you can not control. These movements usually affect the face and tongue. Tardive dyskinesia may not go away, even if you stop taking Zyprexa Relprevv. It may also start after you stop taking Zyprexa Relprevv. Tell your doctor if you get any body movements that you can not control.
- Decreased blood pressure when you change positions, with symptoms of dizziness, fast or slow heartbeat, or fainting.
- Difficulty swallowing, that can cause food or liquid to get into your lungs.
- Seizures: Tell your doctor if you have a seizure during treatment with Zyprexa Relprevv.
- Problems with control of body temperature: You could become very hot, for instance when you exercise a lot or stay in an area that is very hot. It is important for you to drink water to avoid dehydration. Call your doctor right away if you become severely ill and have any of these symptoms of dehydration:
- sweating too much or not at all
- dry mouth
- feeling very hot
- feeling thirsty
- not able to produce urine
Common side effects of Zyprexa Relprevv include: headache, sleepiness or drowsiness, weight gain, dry mouth, diarrhea, nausea, common cold, eating more (increased appetite), vomiting, cough, back pain, or pain at the injection site.
Tell your doctor about any side effect that bothers you or that does not go away.
These are not all the possible side effects with Zyprexa Relprevv. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Zyprexa Relprevv
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
This Medication Guide summarizes the most important information about Zyprexa Relprevv. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Zyprexa Relprevv that was written for healthcare professionals. For more information about Zyprexa Relprevv call 1-800-Lilly-Rx (1-800-545-5979) or visit www.zyprexarelprevv.com.
What are the ingredients in Zyprexa Relprevv?
Active ingredient: olanzapine
Inactive ingredients: carboxymethylcellulose sodium, mannitol, polysorbate 80, sodium hydroxide and/or hydrochloric acid for pH adjustment, and water for injection
Instructions for use for Zyprexa Relprevv
Instructions to Reconstitute and Administer Zyprexa Relprevv
FOR DEEP INTRAMUSCULAR GLUTEAL INJECTION ONLY.
NOT TO BE INJECTED INTRAVENOUSLY OR SUBCUTANEOUSLY.
STEP 1
PREPARING MATERIALS
Convenience kit includes:
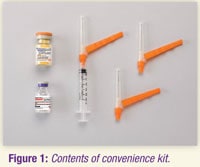
- Vial of Zyprexa Relprevv powder
- 3-mL vial of diluent
- One 3-mL syringe with pre-attached 19-gauge, 1.5-inch (38 mm) Hypodermic Needle-Pro needle with needle protection device
- Two 19-gauge, 1.5-inch (38 mm) Hypodermic Needle-Pro needles with needle protection device
- For obese patients, a 2-inch (50 mm), 19-gauge or larger needle (not included in convenience kit) may be used for administration.
 Zyprexa Relprevv must be suspended using only the diluent supplied in the convenience kit.
Zyprexa Relprevv must be suspended using only the diluent supplied in the convenience kit.
It is recommended that gloves are used when reconstituting, as Zyprexa Relprevv may be irritating to the skin. Flush with water if contact is made with skin.
DETERMINING RECONSTITUTION VOLUME
Refer to the table below to determine the amount of diluent to be added to powder for reconstitution of each vial strength.
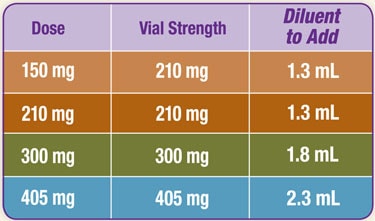
 It is important to note that there is more diluent in the vial than is needed to reconstitute.
It is important to note that there is more diluent in the vial than is needed to reconstitute.
STEP 3
RECONSTITUTING ZYPREXA RELPREVV
Please read the Hypodermic Needle-Pro Instructions for Use before proceeding with Step 3. Failure to follow these instructions may result in a needle stick injury.
3.1 Loosen the powder by lightly tapping the vial.
3.2 Open the prepackaged Hypodermic Needle-Pro syringe and needle with needle protection device.
3.3 Withdraw the pre-determined diluent volume (Step 2) into the syringe.
3.4 Inject the diluent into the powder vial.
3.5 Withdraw air to equalize the pressure in the vial by pulling back slightly on the plunger in the syringe.
3.6 Remove the needle from the vial, holding the vial upright to prevent any loss of material.
3.7 Engage the needle safety device (refer to complete Hypodermic Needle-Pro Instructions for Use).
3.8 Pad a hard surface to cushion impact (see Figure 2). Tap the vial firmly and repeatedly on the surface until no powder is visible.
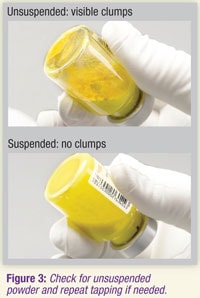
3.9 Visually check the vial for clumps. Unsuspended powder appears as yellow, dry clumps clinging to the vial. Additional tapping may be required if large clumps remain (see Figure 3).
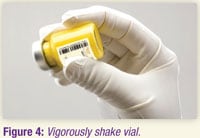
3.10 Shake the vial vigorously until the suspension appears smooth and is consistent in color and texture. The suspended product will be yellow and opaque (see Figure 4).
If foam forms, let vial stand to allow foam to dissipate.
If the product is not used right away, it should be shaken vigorously to re-suspend. Reconstituted Zyprexa Relprevv remains stable at room temperature for up to 24 hours in the vial.
STEP 4
INJECTING ZYPREXA RELPREVV
 IMPORTANT. Before administering the injection, confirm there will be someone to accompany the patient after the 3-hour observation period. If this cannot be confirmed, do not give the injection.
IMPORTANT. Before administering the injection, confirm there will be someone to accompany the patient after the 3-hour observation period. If this cannot be confirmed, do not give the injection.
Refer to the table below to determine the final volume to inject. Suspension concentration is 150 mg/mL Zyprexa Relprevv.
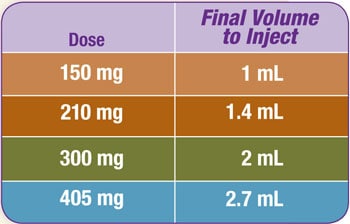
4.1 Attach a new safety needle to the syringe.
4.2 Slowly withdraw the desired amount into the syringe.
 SOME EXCESS PRODUCT WILL REMAIN IN THE VIAL.
SOME EXCESS PRODUCT WILL REMAIN IN THE VIAL.
4.3 Engage the needle safety device and remove the needle from syringe.
4.4 For administration, select the 19-gauge, 1.5-inch (38 mm) Hypodermic Needle-Pro needle with needle protection device. For obese patients, a 2-inch (50 mm), 19-gauge or larger needle (not included in convenience kit) may be used. To help prevent clogging, a 19-gauge or larger needle must be used.
4.5 Attach the new safety needle to the syringe prior to injection. Once the suspension has been removed from the vial, it should be injected immediately.
 FOR DEEP INTRAMUSCULAR GLUTEAL INJECTION ONLY.
FOR DEEP INTRAMUSCULAR GLUTEAL INJECTION ONLY.
DO NOT INJECT INTRAVENOUSLY OR SUBCUTANEOUSLY.
4.6 Select and prepare a site for injection in the gluteal area.
4.7 After insertion of the needle into the muscle,  aspirate for several seconds to ensure that no blood appears. If any blood is drawn into the syringe, discard the syringe and the dose and begin with a new convenience kit. The injection should be performed with steady, continuous pressure.
aspirate for several seconds to ensure that no blood appears. If any blood is drawn into the syringe, discard the syringe and the dose and begin with a new convenience kit. The injection should be performed with steady, continuous pressure.
 DO NOT MASSAGE THE INJECTION SITE.
DO NOT MASSAGE THE INJECTION SITE.
4.8 Engage the needle safety device.
4.9 Dispose of the vials, needles, and syringe appropriately after injection. The vial is for single-use only.



