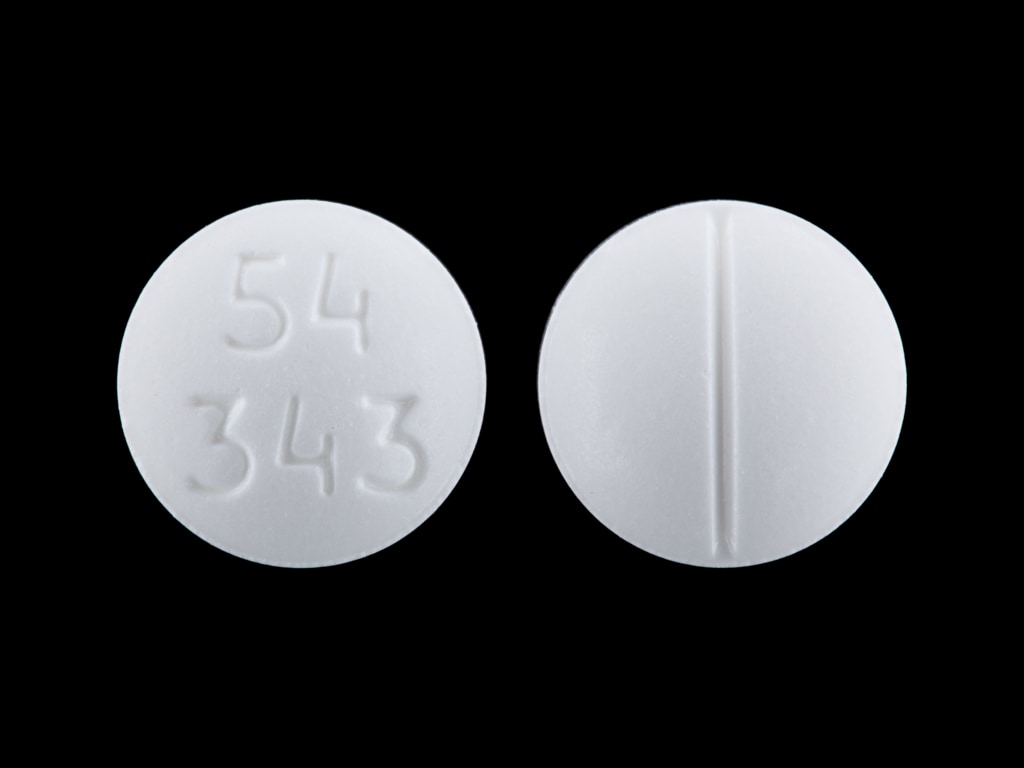
Pill Imprint 54 343
This white round pill with imprint 54 343 on it has been identified as: Prednisone 50 mg.
This medicine is known as prednisone. It is available as a prescription only medicine and is commonly used for Acute Lymphocytic Leukemia, Adrenocortical Insufficiency, Congenital Adrenal Hyperplasia, Allergic Reactions, Allergic Rhinitis, Ankylosing Spondylitis, Aspiration Pneumonia, Asthma, Atopic Dermatitis, Autoimmune Hemolytic Anemia, Berylliosis, Bullous Pemphigoid, Bursitis, Chorioretinitis, Cluster Headaches, Cogan's Syndrome, Conjunctivitis, Allergic, COPD, Corneal Ulcer, Crohn's Disease, Active, Dermatitis Herpetiformis, Dermatomyositis, Diffuse Large B-Cell Lymphoma, Eczema, Epicondylitis, Tennis Elbow, Erythroblastopenia, Fibromyalgia, Giant Cell Arteritis, Gouty Arthritis, Graft Versus Host Disease, Herpes Zoster, Herpes Zoster Iridocyclitis, Hypercalcemia of Malignancy, Immune Thrombocytopenia, Immunosuppression, Inflammatory Bowel Disease, Inflammatory Conditions, Interstitial Lung Disease, Iridocyclitis, Iritis, Juvenile Rheumatoid Arthritis, Keratitis, Leukemia, Lichen Planus, Lichen Sclerosus, Loeffler's Syndrome, Lymphoma, Mixed Connective Tissue Disease, Multiple Sclerosis, Mycosis Fungoides, Nephrotic Syndrome, Neurosarcoidosis, Osteoarthritis, Pemphigoid, Pemphigus, Pharyngitis, Polymyalgia Rheumatica, Polymyositis/Dermatomyositis, Psoriasis, Psoriatic Arthritis, Ramsay Hunt Syndrome, Rheumatoid Arthritis, Sarcoidosis, Scleroderma, Seborrheic Dermatitis, Sinusitis, Skin Rash, Synovitis, Lupus, Systemic Sclerosis, Thrombocytopenia, Toxic Epidermal Necrolysis, Tuberculosis, Extrapulmonary, Tuberculous Meningitis, Ulcerative Colitis, Active, Uveitis, Posterior, Lupus Nephritis, Lichen Planopilaris, Optic Neuritis, Allergies, Amyloidosis, Food Allergies, Immunoglobulin G4-Related Disease.
Details for pill imprint 54 343
- Drug
- Prednisone
- Imprint
- 54 343
- Strength
- 50 mg
- Color
- White
- Shape
- Round
- Size
- 10mm
- Availability
- Prescription only
Pill Classification
- National Drug Code (NDC)
- 000540019 - Roxane Laboratories, Inc.