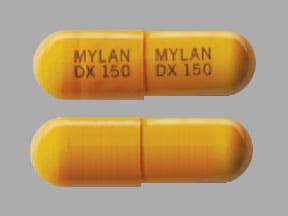
Pill Imprint MYLAN DX 150 MYLAN DX 150
This orange capsule-shape pill with imprint MYLAN DX 150 MYLAN DX 150 on it has been identified as: Doxycycline 150 mg.
This medicine is known as doxycycline. It is available as a prescription only medicine and is commonly used for Acne, Actinomycosis, Amebiasis, Anthrax, Anthrax Prophylaxis, Bacterial Infection, Bartonellosis, Bronchiectasis, Bronchitis, Brucellosis, Bullous Pemphigoid, Cervicitis, Chancroid, Chlamydia Infection, Cholera, Cutaneous Bacillus anthracis, Ehrlichiosis, Enterocolitis, Epididymitis, Sexually Transmitted, Gastroenteritis, Gonococcal Infection, Uncomplicated, Granuloma Inguinale, Inclusion Conjunctivitis, Lyme Disease, Lyme Disease, Arthritis, Lyme Disease, Carditis, Lyme Disease, Erythema Chronicum Migrans, Lyme Disease, Neurologic, Lymphogranuloma Venereum, Malaria, Malaria Prevention, Melioidosis, Mycoplasma Pneumonia, Nongonococcal Urethritis, Ocular Rosacea, Ornithosis, Pelvic Inflammatory Disease, Pemphigoid, Pemphigus, Periodontitis, Plague, Pleural Effusion, Pneumonia, Proctitis, Prostatitis, Psittacosis, Q Fever, Rabbit Fever, Rheumatoid Arthritis, Rickettsial Infection, Rosacea, Skin or Soft Tissue Infection, STD Prophylaxis, Syphilis, Early, Syphilis, Latent, Tertiary Syphilis, Trachoma, Upper Respiratory Tract Infection, Urinary Tract Infection, Lichen Planopilaris, Skin and Structure Infection.
Details for pill imprint MYLAN DX 150 MYLAN DX 150
- Drug
- Doxycycline
- Imprint
- MYLAN DX 150 MYLAN DX 150
- Strength
- 150 mg
- Color
- Orange
- Shape
- Capsule-shape
- Availability
- Prescription only
Pill Classification
- National Drug Code (NDC)
- 003785475 - Mylan Pharmaceuticals Inc.