Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Orilissa: 150 mg [contains carmine]
Orilissa: 200 mg
Pharmacology
Mechanism of Action
Elagolix is a short-acting, nonpeptide, gonadotropin-releasing hormone antagonist that suppresses pituitary and ovarian hormone function in a dose-dependent manner. Concentrations of LH, FSH, and estradiol are decreased during therapy and rapidly return to previous levels once treatment is discontinued. In patients with endometriosis, these actions reduce dysmenorrhea and nonmenstrual pelvic pain (Ng 2017; Struthers 2009; Taylor 2017).
Pharmacokinetics/Pharmacodynamics
Absorption
Rapid (Ng 2017)
Metabolism
Hepatic via CYP3A (major pathway) and CYP2D6, CYP2C8, and UGTs
Excretion
Feces 90%; urine <3%
Onset of Action
FSH, LH, and estradiol suppression: Within hours of day 1 (initial) administration (Ng 2017)
Time to Peak
1 hour
Duration of Action
FSH, LH, and estradiol concentrations return to baseline or higher within 24 to 48 hours after discontinuation (Ng 2017)
Half-Life Elimination
4 to 6 hours
Protein Binding
80%; to human plasma proteins
Use in Specific Populations
Special Populations: Hepatic Function Impairment
Exposure of elagolix is increased by ~3-fold in patients with moderate hepatic impairment and by ~7-fold in patients with severe hepatic impairment (compared to patients with normal hepatic function).
Use: Labeled Indications
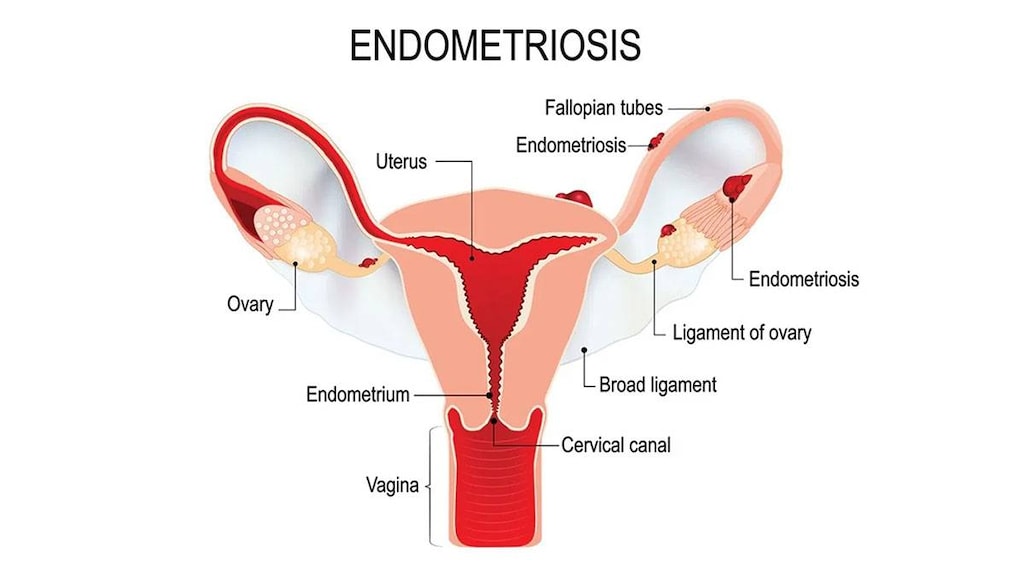
Endometriosis: Management of moderate to severe pain associated with endometriosis.
Contraindications
Pregnancy; known osteoporosis; severe hepatic impairment (Child-Pugh class C); concomitant use of strong organic anion transporting polypeptide (OATP) 1B1 inhibitors (eg, cyclosporine, gemfibrozil)
Canadian labeling: Additional contraindications (not in US labeling): Hypersensitivity to elagolix or any component of the formulation; undiagnosed vaginal bleeding
Dosage and Administration
Dosing: Adult
Note: Treatment should be at the lowest effective dose consistent with treatment goals/severity of symptoms; limit duration of use to avoid potential bone loss.
Endometriosis: Oral: Initial: 150 mg once daily; Maximum treatment duration: 24 months
Endometriosis with dyspareunia: Oral: Initial: Consider an initial dose of 200 mg twice daily; Maximum treatment duration: 6 months
Missed dose: If a dose is missed, the dose should be administered on the same day as soon as remembered and then resume the regular schedule. For patients on 150 mg once daily, administer no more than 1 tablet each day; for patients on 200 mg twice daily, administer no more than 2 tablets each day.
Administration
Oral: Administer at approximately the same time(s) each day, with or without food. Treatment should be initiated within 7 days of menses onset otherwise pregnancy must be excluded prior to therapy.
Dietary Considerations
Consider calcium and vitamin D supplementation.
Storage
Store at 2°C to 30°C (36°F to 86°F).
Drug Interactions
Afatinib: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Afatinib. Management: Reduce afatinib by 10 mg if not tolerated. Some non-US labeling recommends avoiding combination if possible. If used, administer the P-gp inhibitor simultaneously with or after the dose of afatinib. Consider therapy modification
Aprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Betrixaban: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Betrixaban. Management: Decrease the adult betrixaban dose to an initial single dose of 80 mg followed by 40 mg once daily if combined with a P-glycoprotein inhibitor. Consider therapy modification
Bilastine: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Bilastine. Management: Consider alternatives when possible; bilastine should be avoided in patients with moderate to severe renal insufficiency who are receiving p-glycoprotein inhibitors. Consider therapy modification
Brentuximab Vedotin: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Brentuximab Vedotin. Specifically, concentrations of the active monomethyl auristatin E (MMAE) component may be increased. Monitor therapy
Celiprolol: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Celiprolol. Monitor therapy
Clofazimine: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CloZAPine: CYP3A4 Inducers (Weak) may decrease the serum concentration of CloZAPine. Monitor therapy
Colchicine: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Colchicine. Colchicine distribution into certain tissues (e.g., brain) may also be increased. Management: Colchicine is contraindicated in patients with impaired renal or hepatic function who are also receiving a p-glycoprotein inhibitor. In those with normal renal and hepatic function, reduce colchicine dose as directed. See full monograph for details. Consider therapy modification
Conivaptan: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
CYP3A4 Inducers (Strong): May decrease the serum concentration of Elagolix. Monitor therapy
CYP3A4 Inhibitors (Moderate): May decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CYP3A4 Inhibitors (Strong): May increase the serum concentration of Elagolix. Management: Use of the elagolix 200 mg twice daily dose with a strong CYP3A4 inhibitor for longer than 1 month is not recommended. Limit combined use of the elagolix 150 mg once daily dose with a strong CYP3A4 inhibitor to a maximum of 6 months. Consider therapy modification
Dabigatran Etexilate: P-glycoprotein/ABCB1 Inhibitors may increase serum concentrations of the active metabolite(s) of Dabigatran Etexilate. Monitor therapy
Digoxin: Elagolix may increase the serum concentration of Digoxin. Monitor therapy
DOXOrubicin (Conventional): P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of DOXOrubicin (Conventional). Management: Seek alternatives to P-glycoprotein inhibitors in patients treated with doxorubicin whenever possible. One U.S. manufacturer (Pfizer Inc.) recommends that these combinations be avoided. Consider therapy modification
Duvelisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Edoxaban: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Edoxaban. Management: See full monograph for details. Reduced doses are recommended for patients receiving edoxaban for venous thromboembolism in combination with certain P-gp inhibitors. Similar dose adjustment is not recommended for edoxaban use in atrial fibrillation. Consider therapy modification
Erdafitinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Estrogen Derivatives (Contraceptive): May diminish the therapeutic effect of Elagolix. Management: Use an alternative, non-hormonal contraceptive during treatment with elagolix and for at least 1 week following discontinuation of elagolix treatment. Consider therapy modification
Everolimus: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Everolimus. Monitor therapy
Fosaprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fosnetupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fusidic Acid (Systemic): May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Idelalisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Larotrectinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Larotrectinib: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Larotrectinib. Monitor therapy
Lefamulin: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Lefamulin. Management: Avoid concomitant use of lefamulin tablets with P-glycoprotein/ABCB1 inhibitors. If concomitant use is required, monitor for lefamulin adverse effects. Consider therapy modification
Midazolam: Elagolix may decrease the serum concentration of Midazolam. Monitor therapy
MiFEPRIStone: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Minimize doses of CYP3A4 substrates, and monitor for increased concentrations/toxicity, during and 2 weeks following treatment with mifepristone. Avoid cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, and tacrolimus. Consider therapy modification
Naldemedine: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Naldemedine. Monitor therapy
Naloxegol: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Naloxegol. Monitor therapy
Netupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
NiMODipine: CYP3A4 Inducers (Weak) may decrease the serum concentration of NiMODipine. Monitor therapy
OATP1B1/1B3 (SLCO1B1/1B3) Inhibitors: May increase the serum concentration of Elagolix. Avoid combination
Omeprazole: Elagolix may increase the serum concentration of Omeprazole. Management: No action required for omeprazole doses of 40 mg daily or lower. If elagolix is used concomitantly with omeprazole doses higher than 40 mg daily, consider reducing the dose of omeprazole. Consider therapy modification
Palbociclib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
PAZOPanib: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of PAZOPanib. Avoid combination
P-glycoprotein/ABCB1 Substrates: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of P-glycoprotein/ABCB1 Substrates. P-glycoprotein inhibitors may also enhance the distribution of p-glycoprotein substrates to specific cells/tissues/organs where p-glycoprotein is present in large amounts (e.g., brain, T-lymphocytes, testes, etc.). Exceptions: Loperamide. Monitor therapy
Prucalopride: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Prucalopride. Monitor therapy
Ranolazine: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Ranolazine. Monitor therapy
RifAMPin: May increase the serum concentration of Elagolix. Management: Use of the elagolix 200 mg twice daily dose with rifampin is not recommended. Limit combined use of the elagolix 150 mg once daily dose with rifampin to a maximum of 6 months. Consider therapy modification
RifAXIMin: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of RifAXIMin. Monitor therapy
Rosuvastatin: Elagolix may decrease the serum concentration of Rosuvastatin. Monitor therapy
Silodosin: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Silodosin. Monitor therapy
Stiripentol: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Use of stiripentol with CYP3A4 substrates that are considered to have a narrow therapeutic index should be avoided due to the increased risk for adverse effects and toxicity. Any CYP3A4 substrate used with stiripentol requires closer monitoring. Consider therapy modification
Talazoparib: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Talazoparib. Management: These listed exceptions are discussed in detail in separate interaction monographs. Monitor therapy
Tegaserod: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Tegaserod. Monitor therapy
Tolvaptan: May increase the serum concentration of OATP1B1/1B3 (SLCO1B1/1B3) Substrates. Consider therapy modification
Topotecan: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Topotecan. Avoid combination
Ubrogepant: CYP3A4 Inducers (Weak) may decrease the serum concentration of Ubrogepant. Management: Use an initial ubrogepant dose of 100 mg and second dose (if needed) of 100 mg when used with a weak CYP3A4 inducer. Consider therapy modification
Ubrogepant: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Ubrogepant. Management: Use an initial ubrogepant dose of 50 mg and second dose (if needed) of 50 mg when used with a P-gp inhibitor. Consider therapy modification
Venetoclax: P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of Venetoclax. Management: Consider a venetoclax dose reduction by at least 50% in patients requiring concomitant treatment with P-glycoprotein (P-gp) inhibitors. Consider therapy modification
VinCRIStine (Liposomal): P-glycoprotein/ABCB1 Inhibitors may increase the serum concentration of VinCRIStine (Liposomal). Avoid combination
Adverse Reactions
>10%:
Central nervous system: Headache (17% to 20%)
Dermatologic: Night sweats (≤46%)
Endocrine & metabolic: Amenorrhea (4% to 57%), hot flash (≤46%)
Gastrointestinal: Nausea (16%)
Neuromuscular & skeletal: Decreased bone mineral density (≤21%)
1% to 10%:
Central nervous system: Insomnia (6% to 9%), depressed mood (≤6%), depression (≤6%), emotional lability (≤6%), lacrimation (≤6%; including tearfulness), mood changes (≤6%), anxiety (5%), dizziness (≥3% to <5%), irritability (≥3% to <5%)
Endocrine & metabolic: Decreased libido (≥3% to <5%), weight gain (≥3% to <5%)
Gastrointestinal: Abdominal pain (≥3% to <5%), constipation (≥3% to <5%), diarrhea (≥3% to <5%)
Hepatic: Increased serum alanine aminotransferase (≥3 x ULN: 1%)
Neuromuscular & skeletal: Arthralgia (5%)
Frequency not defined:
Endocrine & metabolic: Increased HDL cholesterol, increased LDL cholesterol, increased serum cholesterol, increased triglycerides
Genitourinary: Menstrual flow (reduction in the amount, intensity, or duration of menstrual bleeding)
<1%, postmarketing, and/or case reports: Appendicitis, back pain, suicidal ideation
Warnings/Precautions
Concerns related to adverse effects:
- Bleeding irregularities: Menstrual bleeding patterns may change, causing a decrease in the amount, intensity, or duration of bleeding. Changes in bleeding patterns may alter the ability to detect pregnancy. Pregnancy testing should be conducted if pregnancy is suspected; discontinue use if pregnancy is confirmed.
- BMD loss: A dose-dependent decrease in BMD is associated with elagolix exposure. The risk of BMD loss is increased with duration of use and may not be completely reversible following discontinuation of elagolix. Evaluate patients for osteoporosis risk factors (including a history of low-trauma fracture) prior to therapy. Consider supplementation with calcium and vitamin D. Duration of treatment should be limited to prevent BMD loss. Do not use in patients with known osteoporosis.
- Depression: Elagolix may increase the risk of depression and mood changes; risk may be increased in patients with a history of suicidality or depression. Suicidal ideation/behavior has been reported. Promptly evaluate new or worsening psychiatric symptoms and refer to a mental health care professional if appropriate. Patients should seek immediate medical attention for suicidal ideation and behavior. Consider risks and benefits of therapy if mood disturbances occur.
- Hepatic impairment: Dose-dependent elevations of ALT ≥3 times the upper limit of normal may occur; the lowest effective dose should be used. Promptly evaluate elevations in transaminases to assess risks versus benefits of continuing therapy. Dose adjustment is required in patients with moderate hepatic impairment; do not use in severe hepatic impairment.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Monitoring Parameters
Pregnancy status prior to therapy; pregnancy test during therapy (if pregnancy is suspected); liver function tests; monitor mental status (for signs/symptoms of depression, suicidal ideation); consider BMD after 12 months (Surrey 2018)
Pregnancy
Pregnancy Considerations
Use is contraindicated during pregnancy.
Information related to inadvertent exposure in pregnancy is limited. Elagolix may increase the risk of early pregnancy loss.
Evaluate pregnancy status prior to treatment. Exclude pregnancy prior to treatment or start elagolix within 7 days from the onset of menses. Treatment with elagolix may reduce the amount, intensity, or duration of menstrual bleeding, which may make it more difficult to recognize pregnancy. Perform pregnancy testing if pregnancy is suspected during elagolix therapy; discontinue elagolix if pregnancy occurs.
Ovulation is not fully suppressed during elagolix therapy (Taylor 2017). Nonhormonal contraceptives should be used during therapy and for 1 week after elagolix treatment is discontinued.
Data collection to monitor pregnancy and infant outcomes following exposure to elagolix is ongoing. Health care providers are encouraged to enroll females exposed to elagolix during pregnancy in the pregnancy registry (833-782-7241).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience menstrual changes, hot flash, night sweats, headache, nausea, trouble sleeping, or joint pain. Have patient report immediately to prescriber signs of liver problems (dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin), signs of depression (thoughts of suicide, anxiety, emotional instability, or confusion), mood changes, behavioral changes, or anxiety (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.