Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Tablet, Oral:
Camila: 0.35 mg
Camila: 0.35 mg [contains corn starch, fd&c red #40 aluminum lake]
Deblitane: 0.35 mg [contains fd&c blue #2 aluminum lake, fd&c red #40 aluminum lake, fd&c yellow #10 aluminum lake, soybean lecithin]
Errin: 0.35 mg [contains corn starch, fd&c yellow #10 aluminum lake]
Heather: 0.35 mg [contains fd&c yellow #10 aluminum lake, fd&c yellow #6 aluminum lake]
Incassia: 0.35 mg [contains corn starch, fd&c yellow #10 aluminum lake, fd&c yellow #6 aluminum lake]
Jencycla: 0.35 mg [contains brilliant blue fcf (fd&c blue #1), fd&c yellow #10 (quinoline yellow)]
Jolivette: 0.35 mg [DSC]
Lyza: 0.35 mg [contains corn starch, fd&c yellow #10 (quinoline yellow)]
Nor-QD: 0.35 mg [DSC]
Nora-BE: 0.35 mg
Norlyda: 0.35 mg
Norlyroc: 0.35 mg
Ortho Micronor: 0.35 mg [contains corn starch, fd&c yellow #10 aluminum lake]
Sharobel: 0.35 mg [contains fd&c blue #1 aluminum lake, fd&c yellow #6 aluminum lake, soybean lecithin]
Tulana: 0.35 mg [contains corn starch, fd&c yellow #10 aluminum lake, fd&c yellow #6 aluminum lake]
Generic: 0.35 mg
Tablet, Oral, as acetate:
Aygestin: 5 mg [scored]
Generic: 5 mg
Pharmacology
Mechanism of Action
Once absorbed, systemic disposition of norethindrone acetate (NETA) and norethindrone (NET) is the same.
NET is used in preparations for progestin-only contraception. NET suppresses ovulation, thickens cervical mucus (which inhibits sperm penetration), alters follicle-stimulating hormone (FSH) and luteinizing hormone (LH) concentrations, slows the movement of ovum through the fallopian tubes, and alters the endometrium.
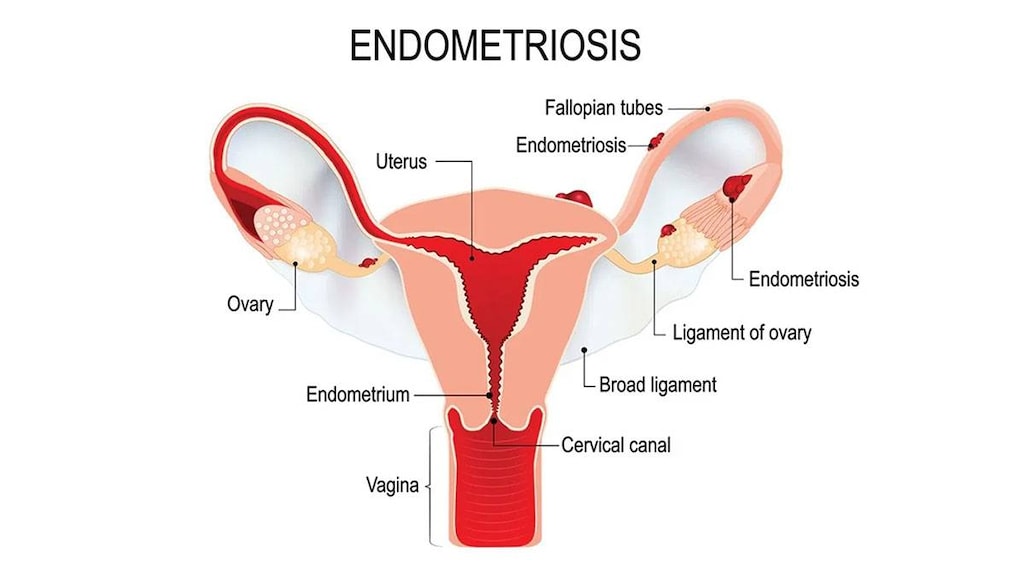
Progestogens, such as NETA in the doses used for abnormal uterine bleeding, amenorrhea, and endometriosis, lead to atrophy of the endometrial tissue. They may also suppress new growth and implantation. Pain associated with endometriosis is decreased. When treating endometriosis, NETA may be used in combination with gonadotropin-releasing hormone agonists to decrease side effects from hypoestrogenism (ASRM 2014).
Pharmacokinetics/Pharmacodynamics
Absorption
Oral: Rapidly absorbed
Distribution
Vd: 4 L/kg
Metabolism
Oral: Norethindrone acetate is deacetylated to norethindrone; norethindrone undergoes hepatic reduction and conjugation; orally administered norethindrone is subject to first-pass effect (Orme 1983). In addition to forming glucuronide and sulfide conjugates, norethindrone is also metabolized to ethinyl estradiol (Kuhnz 1997; Orme 1983)
Excretion
Urine (>50% as metabolites); feces (20% to 40% as metabolites)
Time to Peak
~2 hours (varies by dose and use of concomitant estrogen (Orme 1983)
Half-Life Elimination
~8 to 9 hours
Protein Binding
61% to albumin, 36% to sex hormone-binding globulin (SHBG); SHBG capacity affected by plasma ethinyl estradiol levels (Orme 1983)
Use: Labeled Indications
Abnormal uterine bleeding (norethindrone acetate): Treatment of abnormal uterine bleeding due to hormonal imbalance in absence of organic pathology, such as submucous fibroids or uterine cancer
Amenorrhea, secondary (norethindrone acetate): Treatment of secondary amenorrhea
Contraception (norethindrone): Prevention of pregnancy
Endometriosis (norethindrone acetate): Treatment of endometriosis
Limitations of use:
Norethindrone is not indicated for emergency contraception.
Norethindrone acetate is not indicated for use with estrogen therapy in postmenopausal women for endometrial protection.
Contraindications
Hypersensitivity to norethindrone or any component of the formulation; hepatic impairment or disease; breast cancer (known, suspected, or history of); undiagnosed abnormal genital bleeding; pregnancy
Norethindrone: Additional contraindications: Benign or malignant liver tumors
Norethindrone acetate:
Additional contraindications: DVT or PE (current or history of); active or recent history of arterial thromboembolic disease (eg, stroke, MI); as a diagnostic test for pregnancy
Additional contraindications in Canadian labeling: Estrogen or progestin dependent malignant tumor; partial or complete vision loss due to ophthalmic vascular disease; missed abortion
Dosage and Administration
Dosing: Adult
Abnormal uterine bleeding and amenorrhea: Females: Oral: Norethindrone acetate: 2.5 to 10 mg/day for 5 to 10 days. Secretory transformation of the endometrium will occur when adequately primed with endogenous or exogenous estrogen. Withdrawal bleeding may be expected within 3 to 7 days after discontinuing norethindrone acetate.
Contraception: Females: Oral: Norethindrone: 0.35 mg every day (no missed days)
Initial dose: Start on first day of menstrual period or the day after a miscarriage or abortion. If switching from a combined oral contraceptive, begin the day after finishing the last active combined tablet.
Missed dose: Take as soon as remembered. A back up method of contraception should be used for 48 hours if dose is taken ≥3 hours late.
Additional contraception dosing considerations (Curtis 2016a):
Initiation of therapy: May be started at any time in the menstrual cycle once it is determined that the woman is not pregnant. Back-up contraception is not needed if started within 5 days of onset of menstruation. If started >5 days after the onset of menstruation or at any time in a women experiencing amenorrhea (not postpartum), back up contraception should be used for 2 days.
Switching from a different contraceptive to a progestin-only contraceptive: May be started at any time if it is determined that the woman is not pregnant. Unless the woman abstains from sexual intercourse, a backup method of contraception is needed if it has been >5 days since menstrual bleeding has begun. When an additional method of contraception is needed, consider continuing the woman’s previous method for 2 days after starting the progestin-only contraceptive.
Switching from an IUD to a progestin-only contraceptive: Continue the IUD for at least 2 days after the progestin-only contraceptive is started or advise the woman to abstain from sexual intercourse or use a barrier contraceptive for 7 days before removing the IUD. Alternately, an emergency contraceptive may be used at the time of IUD removal.
Endometriosis: Females: Oral: Norethindrone acetate: 5 mg/day for 14 days; increase at increments of 2.5 mg/day every 2 weeks to reach 15 mg/day; continue for 6 to 9 months or until breakthrough bleeding demands temporary termination
Dosing: Pediatric
Abnormal uterine bleeding and amenorrhea (secondary): Postmenarche females: Limited data in postmenarche children: Norethindrone acetate: Oral: 2.5 to 10 mg once daily for 5 to 12 days each month; most pediatric experts suggest 5 mg once daily for 12 days per month (Kliegman 2011; Kliegman 2016). Secretory transformation of the endometrium will occur when adequately primed with endogenous or exogenous estrogen. Withdrawal bleeding may be expected within 3 to 7 days after discontinuing norethindrone acetate.
Contraception: Postmenarche adolescent females: Norethindrone: Oral: 0.35 mg once daily every day (no missed days)
Initial dose: Start on first day of menstrual period or the day after a miscarriage or abortion. If switching from a combined oral contraceptive, begin the day after finishing the last active combined tablet.
Missed dose: Take as soon as remembered. A back-up method of contraception should be used for 48 hours if dose is taken ≥3 hours late.
Additional contraception dosing considerations (Curtis 2016a):
Initiation of therapy: May be started at any time in the menstrual cycle once it is determined that the female is not pregnant. Back-up contraception is not needed if started within 5 days of onset of menstruation. If started >5 days after the onset of menstruation or at any time in a female experiencing amenorrhea (not postpartum), back up contraception should be used for 2 days.
Switching from a different contraceptive to a progestin-only contraceptive: May be started at any time if it is determined that the female is not pregnant. Unless the female abstains from sexual intercourse, a back-up method of contraception is needed if it has been >5 days since menstrual bleeding has begun. When an additional method of contraception is needed, consider continuing the woman’s previous method for 2 days after starting the progestin-only contraceptive.
Switching from an IUD to a progestin-only contraceptive: Continue the IUD for at least 2 days after the progestin-only contraceptive is started or advise the female to abstain from sexual intercourse or use a barrier contraceptive for 7 days before removing the IUD. Alternately, an emergency contraceptive may be used at the time of IUD removal.
Endometriosis: Postmenarche females ≥10 years: Limited data in children 10 to 12 years (Kaser 2012): Norethindrone acetate: Oral: 5 mg once daily for 14 days; increase at increments of 2.5 mg/day every 2 weeks to reach target maintenance of 15 mg/day; continue for 6 to 9 months or until breakthrough bleeding demands temporary termination
Administration
For oral administration. Administer at the same time each day.
When used for the prevention of pregnancy, a backup method of contraception should be used for 48 hours if dose is missed or taken ≥3 hours late. If vomiting or severe diarrhea occur within 3 hours of taking a dose, take another dose as soon as possible, then continue taking one dose daily and use a backup method of contraception (or avoid sexual intercourse) until 2 days after vomiting or diarrhea have resolved. Emergency contraception should be considered in the event of unprotected intercourse (Curtis 2016a).
Dietary Considerations
Should be taken at same time each day.
Storage
Store at controlled room temperature.
Norethindrone Images
Drug Interactions
Acitretin: May diminish the therapeutic effect of Progestins (Contraceptive). Contraceptive failure is possible. Management: Given the potential for progestin-only preparations to fail to prevent pregnancy during acitretin therapy, such products should not be relied upon. Alternative, nonhormonal forms of contraception must be employed during acitretin therapy. Consider therapy modification
Anticoagulants: Progestins may diminish the therapeutic effect of Anticoagulants. More specifically, the potential prothrombotic effects of some progestins and progestin-estrogen combinations may counteract anticoagulant effects. Management: Carefully weigh the prospective benefits of progestins against the potential increased risk of procoagulant effects and thromboembolism. Use is considered contraindicated under some circumstances. Refer to related guidelines for specific recommendations. Consider therapy modification
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Aprepitant: May decrease the serum concentration of Progestins (Contraceptive). Management: Alternative or additional methods of contraception should be used both during treatment with aprepitant or fosaprepitant and for at least one month following the last aprepitant/fosaprepitant dose. Consider therapy modification
Artemether: May decrease the serum concentration of Progestins (Contraceptive). Management: Consider the use of an alternative (i.e., non-hormonal) means of contraception in all women of childbearing potential who are using artemether. Consider therapy modification
Atazanavir: May increase the serum concentration of Progestins (Contraceptive). However, atazanavir may lead to decreased ethinyl estradiol concentrations and decreased effectiveness of oral contraceptive products. Management: Consider an alternative or additional method of contraception, particularly with combined estrogen/progestin products. Depot medroxyprogesterone acetate may be used without a need for additional contraception. Consider therapy modification
Barbiturates: May diminish the therapeutic effect of Progestins (Contraceptive). Contraceptive failure is possible. Management: Use of alternative, nonhormonal contraceptives is recommended. Consider therapy modification
Bexarotene (Systemic): May decrease the serum concentration of Progestins (Contraceptive). Management: Women of childbearing potential receiving bexarotene should use two reliable forms of contraception (including at least one nonhormonal form). Consider therapy modification
Bile Acid Sequestrants: May decrease the serum concentration of Progestins (Contraceptive). Management: Administer oral progestin-containing contraceptives at least 1 to 4 hours prior to or 4 to 6 hours after administration of a bile acid sequestrant. Consider therapy modification
Bosentan: May decrease the serum concentration of Progestins (Contraceptive). Management: Use an alternative (i.e., non-hormonal) means of contraception for all women of childbearing potential who are using bosentan, and do not rely on hormonal contraceptives alone. Consider therapy modification
Brigatinib: May decrease the serum concentration of Progestins (Contraceptive). Management: Females of childbearing potential should use an alternative, non-hormonal contraceptive during brigatinib therapy and for at least 4 months after the final brigatinib dose. Consider therapy modification
C1 inhibitors: Progestins may enhance the thrombogenic effect of C1 inhibitors. Monitor therapy
CarBAMazepine: May diminish the therapeutic effect of Progestins (Contraceptive). Contraceptive failure is possible. Management: Use of alternative, nonhormonal contraceptives is recommended. Consider therapy modification
Carfilzomib: May enhance the thrombogenic effect of Progestins (Contraceptive). Management: Consider alternative, non-hormonal methods of contraception in patients requiring therapy with carfilzomib. Consider therapy modification
Cenobamate: May decrease the serum concentration of Hormonal Contraceptives. Management: Women should use additional or alternative non-hormonal birth control while taking cenobamate. Consider therapy modification
Cladribine: May diminish the therapeutic effect of Hormonal Contraceptives. Management: Women using systemically acting hormonal contraceptives should add a barrier method during cladribine dosing and for at least 4 weeks after the last dose in each treatment course. Consider therapy modification
CloBAZam: May decrease the serum concentration of Progestins (Contraceptive). Consider therapy modification
Cobicistat: May increase the serum concentration of Progestins (Contraceptive). Management: Consider an alternative, nonhormone-based contraceptive in patients receiving cobicistat-containing products. Drospirenone is specifically contraindicated with atazanavir and cobicistat. Consider therapy modification
Colesevelam: May decrease the serum concentration of Norethindrone. Management: Oral contraceptives containing ethinyl estradiol and norethindrone should be administered at least 4 hours before colesevelam. Consider therapy modification
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May increase the metabolism of CYP3A4 Substrates (High risk with Inducers). Management: Consider an alternative for one of the interacting drugs. Some combinations may be specifically contraindicated. Consult appropriate manufacturer labeling. Consider therapy modification
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Dabrafenib: May decrease the serum concentration of Progestins (Contraceptive). Management: Females of reproductive potential should use an alternative, highly effective, non-hormonal means of contraception during and at least 2 weeks (dabrafenib alone) or 4 months (dabrafenib + trametinib) after discontinuation of dabrafenib treatment. Consider therapy modification
Darunavir: May decrease the serum concentration of Norethindrone. Consider therapy modification
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Efavirenz: May decrease the serum concentration of Progestins (Contraceptive). Management: Use an alternative or additional method of contraception due to possibly decreased contraceptive effectiveness. Injected depot medroxyprogesterone acetate does not appear to participate in this interaction. Consider therapy modification
Elexacaftor, Tezacaftor, and Ivacaftor: Hormonal Contraceptives may enhance the adverse/toxic effect of Elexacaftor, Tezacaftor, and Ivacaftor. Specifically, the risk for rash may be increased. Monitor therapy
Encorafenib: May decrease the serum concentration of Progestins (Contraceptive). Avoid combination
Enzalutamide: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Concurrent use of enzalutamide with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP3A4 substrate should be performed with caution and close monitoring. Consider therapy modification
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Eslicarbazepine: May decrease the serum concentration of Progestins (Contraceptive). Management: Alternative, non-hormonal means of birth control should be considered for women of child-bearing potential. Consider therapy modification
Exenatide: May decrease the serum concentration of Progestins (Oral Contraceptive). Management: Administer oral contraceptives at least one hour prior to exenatide. Consider therapy modification
Felbamate: May decrease the serum concentration of Progestins (Contraceptive). Management: Contraceptive failure is possible. Use of an alternative, nonhormonal method of contraception is recommended. Consider therapy modification
Flibanserin: Progestins (Contraceptive) may increase the serum concentration of Flibanserin. Monitor therapy
Fosamprenavir: Progestins (Contraceptive) may decrease serum concentrations of the active metabolite(s) of Fosamprenavir. Fosamprenavir may decrease the serum concentration of Progestins (Contraceptive). Management: Consider using an alternative or additional means of contraception. Injected depot medroxyprogesterone acetate may be used without a need for additional contraception. Consider therapy modification
Fosaprepitant: May decrease the serum concentration of Progestins (Contraceptive). The active metabolite aprepitant is likely responsible for this effect. Management: Alternative or additional methods of contraception should be used both during treatment with aprepitant or fosaprepitant and for at least one month following the last aprepitant/fosaprepitant dose. Consider therapy modification
Fosphenytoin: May diminish the therapeutic effect of Progestins (Contraceptive). Contraceptive failure is possible. Management: Contraceptive failure is possible. Use of an alternative, nonhormonal contraceptive is recommended. Consider therapy modification
Griseofulvin: May diminish the therapeutic effect of Progestins (Contraceptive). Contraceptive failure is possible. Avoid combination
Herbs (Progestogenic Properties) (eg, Bloodroot, Yucca): May enhance the adverse/toxic effect of Progestins. Monitor therapy
Ivosidenib: May decrease the serum concentration of Progestins (Contraceptive). Management: Consider alternative methods of contraception (ie, non-hormonal) in patients receiving ivosidenib. Consider therapy modification
Ixazomib: May decrease the serum concentration of Progestins (Contraceptive). More specifically, use of ixazomib with dexamethasone may decrease the serum concentrations of contraceptive progestins. Management: Patients of childbearing potential should use a nonhormonal barrier contraceptive during and 90 days following ixazomib treatment. Avoid combination
LamoTRIgine: May decrease the serum concentration of Progestins (Contraceptive). Monitor therapy
Lesinurad: May decrease the serum concentration of Progestins (Contraceptive). Management: Use of an additional, nonhormonal contraceptive is recommended in patients being treated with lesinurad who desire effective contraception. Consider therapy modification
Lixisenatide: May decrease the serum concentration of Progestins (Contraceptive). Management: Administer oral contraceptives 1 hour before or at least 11 hours after administration of lixisenatide. Consider therapy modification
Lopinavir: May decrease the serum concentration of Progestins (Contraceptive). Lopinavir may increase the serum concentration of Progestins (Contraceptive). Management: Consider using an alternative or additional means of contraception. Injected depot medroxyprogesterone acetate and etonogestrel implants may be used without a need for additional contraception. Consider therapy modification
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
Lumacaftor and Ivacaftor: May decrease the serum concentration of Hormonal Contraceptives. Management: Do not rely on hormone-based contraceptives with concurrent use of lumacaftor/ivacaftor; an alternative, non-hormonal, method of contraception should be used if this combination is required. Consider therapy modification
Metreleptin: May decrease the serum concentration of Progestins (Contraceptive). Metreleptin may increase the serum concentration of Progestins (Contraceptive). Monitor therapy
MiFEPRIStone: May diminish the therapeutic effect of Progestins (Contraceptive). MiFEPRIStone may increase the serum concentration of Progestins (Contraceptive). Management: Women of childbearing potential should use an effective, nonhormonal means of contraception during and 4 weeks following mifepristone treatment. Consider therapy modification
Mitotane: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Doses of CYP3A4 substrates may need to be adjusted substantially when used in patients being treated with mitotane. Consider therapy modification
Mycophenolate: May decrease the serum concentration of Progestins (Contraceptive). Management: Use of an additional or alternative (nonhormonal) method of contraception should be considered. Consider therapy modification
Nelfinavir: May decrease the serum concentration of Progestins (Contraceptive). Management: Use an alternative or additional method of contraception due to possibly decreased contraceptive effectiveness. Injected depot medroxyprogesterone acetate does not appear to participate in this interaction. Consider therapy modification
OXcarbazepine: May decrease the serum concentration of Progestins (Contraceptive). Management: Contraceptive failure is possible. Use of an additional or alternative, nonhormonal method of contraception is recommended. Consider therapy modification
Perampanel: May decrease the serum concentration of Progestins (Contraceptive). Management: Patients should use an alternative, nonhormonal-based form of contraception both during the concurrent use of perampanel and for 1 month after discontinuing perampanel. Consider therapy modification
Phenytoin: May diminish the therapeutic effect of Progestins (Contraceptive). Contraceptive failure is possible. Management: Contraceptive failure is possible. Use of an alternative, nonhormonal contraceptive is recommended. Consider therapy modification
Pitolisant: May decrease the serum concentration of Hormonal Contraceptives. Management: Patients using hormonal contraception should be advised to use an alternative non-hormonal contraceptive method during treatment with pitolisant and for at least 21 days after discontinuation of pitolisant treatment. Consider therapy modification
Pomalidomide: Progestins may enhance the thrombogenic effect of Pomalidomide. Management: Canadian pomalidomide labeling recommends caution with use of hormone replacement therapy and states that hormonal contraceptives are not recommended. US pomalidomide labeling does not contain these specific recommendations. Consider therapy modification
Primidone: May diminish the therapeutic effect of Progestins (Contraceptive). Contraceptive failure is possible. Management: Use of alternative, nonhormonal contraceptives is recommended. Consider therapy modification
Retinoic Acid Derivatives: May diminish the therapeutic effect of Progestins (Contraceptive). Retinoic Acid Derivatives may decrease the serum concentration of Progestins (Contraceptive). Management: Two forms of effective contraception should be used in patients receiving retinoic acid derivatives. Microdosed progesterone-only preparations (ie, minipills that do not contain estrogen) are considered an inadequate method of contraception. Exceptions: Adapalene; Alitretinoin (Topical); Bexarotene (Topical); Tretinoin (Topical). Consider therapy modification
Rifamycin Derivatives: May decrease the serum concentration of Progestins (Contraceptive). Contraceptive failure is possible. Management: Contraceptive failure is possible. Use of an alternative, nonhormonal contraceptive is recommended. Consider therapy modification
Rufinamide: May decrease the serum concentration of Norethindrone. Consider therapy modification
Saquinavir: May decrease the serum concentration of Progestins (Contraceptive). Management: Use an alternative or additional method of contraception due to possibly decreased contraceptive effectiveness. Injected depot medroxyprogesterone acetate does not appear to participate in this interaction. Consider therapy modification
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Selegiline: Progestins (Contraceptive) may increase the serum concentration of Selegiline. Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
St John's Wort: May diminish the therapeutic effect of Progestins (Contraceptive). Contraceptive failure is possible. Management: Consider using a product other than St John's wort. Contraceptive failure is possible. Use of an alternative, nonhormonal contraceptive is recommended. Consider therapy modification
Sugammadex: May decrease the serum concentration of Progestins (Contraceptive). Management: Patients receiving any hormonal contraceptive (oral or non-oral) should use an additional, nonhormonal contraceptive method during and for 7 days following sugammadex treatment. Consider therapy modification
Tazemetostat: May decrease the serum concentration of Hormonal Contraceptives. Management: Women of reproductive potential should use a non-hormonal contraceptive method during treatment with tazemetostat and for 6 months after. Men with female partners should use contraception during treatment and for 3 months after. Consider therapy modification
Tetrahydrocannabinol and Cannabidiol: May decrease the serum concentration of Hormonal Contraceptives. Management: Women using hormonal contraceptives should consider adding a barrier contraceptive due to the potential for tetrahydrocannabinol and cannabidiol to decrease concentrations and effectiveness of hormonal contraceptives. Consider therapy modification
Thalidomide: Progestins (Contraceptive) may enhance the thrombogenic effect of Thalidomide. Monitor therapy
Tipranavir: May increase the serum concentration of Progestins (Contraceptive). Management: Use an alternative or additional method of contraception due to possibly decreased contraceptive effectiveness. Injected depot medroxyprogesterone acetate does not appear to participate in this interaction. Consider therapy modification
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Topiramate: May decrease the serum concentration of Progestins (Contraceptive). Management: Caution patients that this combination may be associated with reduced contraceptive effectiveness. Consider adding an additional (non-hormonal) contraceptive method. Consider therapy modification
Tranexamic Acid: Progestins (Contraceptive) may enhance the thrombogenic effect of Tranexamic Acid. Avoid combination
Triazolam: Hormonal Contraceptives may increase the serum concentration of Triazolam. Monitor therapy
Ulipristal: Progestins may diminish the therapeutic effect of Ulipristal. Ulipristal may diminish the therapeutic effect of Progestins. Management: Ulipristal for uterine fibroids (Canadian indication): avoid progestins within 12 days of stopping ulipristal; as emergency contraceptive (U.S. indication): avoid progestins within 5 days of stopping ulipristal. Avoid combination
Vitamin K Antagonists (eg, warfarin): Progestins (Contraceptive) may diminish the anticoagulant effect of Vitamin K Antagonists. In contrast, enhanced anticoagulant effects have also been noted with some products. Management: When possible, concomitant hormonal contraceptives and coumarin derivatives should be avoided in order to eliminate the risk of thromboembolic disorders. Consider using an alternative, nonhormonal contraceptive. Consider therapy modification
Voriconazole: May increase the serum concentration of Progestins (Contraceptive). Progestins (Contraceptive) may increase the serum concentration of Voriconazole. Monitor therapy
Test Interactions
Reduced response to metyrapone test. Progestin-only contraceptives may affect sex hormone binding globulin (decrease) or thyroxine concentrations (decrease; because of decreased thyroid binding globulin).
Adverse Reactions
Frequency not defined.
Cardiovascular: Cerebral embolism, cerebral thrombosis, deep vein thrombosis, edema, pulmonary embolism, retinal thrombosis
Central nervous system: Depression, dizziness, fatigue, headache, insomnia, migraine, emotional lability, nervousness
Dermatologic: Acne vulgaris, alopecia, chloasma, pruritus, skin rash, urticaria
Endocrine & metabolic: Amenorrhea, hirsutism, hypermenorrhea, menstrual disease, weight gain
Gastrointestinal: Abdominal pain, nausea, vomiting
Genitourinary: Breakthrough bleeding, breast hypertrophy, breast tenderness, cervical erosion, change in cervical secretions, decreased lactation, genital discharge, mastalgia, spotting, vaginal hemorrhage
Hypersensitivity: Anaphylaxis, hypersensitivity
Hepatic: Cholestatic jaundice, hepatitis, abnormal hepatic function tests
Neuromuscular & skeletal: Arm pain, leg pain
Ophthalmic: Optic neuritis (with or without vision loss)
Warnings/Precautions
Concerns related to adverse effects:
- Bleeding: Irregular menstrual bleeding patterns are common with progestin-only contraceptives; nonpharmacologic causes of abnormal bleeding should be ruled out.
- Breast cancer: The use of combination hormonal contraceptives has been associated with a slight increase in the frequency of breast cancer, however studies are not consistent. Data is insufficient to determine if progestin-only contraceptives also increase this risk. Norethindrone and norethindrone acetate are contraindicated in women with breast cancer.
- Delayed follicular atresia/ovarian cysts: If follicular development occurs following use for contraception, follicles may grow and enlarge beyond the size attained in a normal cycle. May be asymptomatic or can be associated with mild abdominal pain; surgical intervention is rarely required.
- Ectopic pregnancy: The possibility of ectopic pregnancy following use of a progestin-only contraceptive should be considered in patients with lower abdominal pain.
- Lipid effects: May have adverse effects on lipid metabolism; use caution in women with hyperlipidemias.
- Visual abnormalities: Norethindrone acetate: Discontinue if migraine, loss of vision, proptosis, diplopia, or other visual disturbances occur; discontinue permanently if papilledema or retinal vascular lesions are observed on examination.
Disease-related concerns:
- Cardiovascular disease: Norethindrone acetate: Risk factors for cardiovascular disorders include diabetes mellitus, hypercholesterolemia, hypertension, SLE, obesity, tobacco use, and/or history of venous thromboembolism (VTE). Risk factors should be managed appropriately.
- Depression: Norethindrone acetate: Use with caution in patients with depression. Progestin only contraceptive tablets may be used in women with depression (Curtis 2016b).
- Diabetes: May have adverse effects on glucose tolerance; use caution in women with diabetes. Progestin only contraceptive tablets may be used in women with diabetes (Curtis 2016b).
- Diseases exacerbated by fluid retention: Norethindrone acetate: Use with caution in patients with diseases which may be exacerbated by fluid retention, including asthma, epilepsy, or cardiac or renal dysfunction.
- Hepatic adenomas: Extremely rare hepatic adenomas and focal nodular hyperplasia resulting in fatal intra-abdominal hemorrhage have been reported in association with long-term combination oral contraceptive use. Data is insufficient to determine if progestin-only contraceptives also increase this risk. Use as a contraceptive is contraindicated in women with hepatic tumors.
- Migraine: Use with caution in patients with a history of migraine. Progestin-only contraceptive tablets may be used in women with a history of headache or migraine; new headaches or changes in headaches should be evaluated (Curtis 2016b).
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pediatric: Not for use prior to menarche.
- Smokers: Progestin-only contraceptives may be used in women who smoke (Curtis 2016b). Because of an increased risk of cardiovascular disease, women using oral contraceptives should be strongly advised not to smoke.
Other warnings/precautions:
- Appropriate use: Norethindrone: Progestin-only contraceptives contain less progestin than contained in estrogen/progestin–combined contraceptives. Risks associated with estrogen/progestin contraceptives should be considered for progestin-only products.
- HIV infection protection: Progestin-only contraceptives do not protect against HIV infection or other sexually transmitted diseases.
- Laboratory changes: The use of estrogens and/or progestins may change the results of some laboratory tests (eg, coagulation factors, lipids, glucose tolerance, binding proteins). The dose, route, and the specific estrogen/progestin influence these changes. In addition, personal risk factors (eg, cardiovascular disease, smoking, diabetes, age) also contribute to adverse events; use of specific products may be contraindicated in women with certain risk factors.
Monitoring Parameters
Norethindrone: Contraception: Assessment of pregnancy status (prior to therapy); weight (optional; BMI at baseline may be helpful to monitor changes during therapy); assess potential health status changes at routine visits (Curtis 2016a).
Norethindrone acetate: Monitor patient for vision changes; signs or symptoms of depression; glycemic control in patients with diabetes; lipid profiles in patients being treated for hyperlipidemias.
Regardless of indication, adequate diagnostic measures, including endometrial sampling, if indicated, should be performed to rule out malignancy in all cases of undiagnosed abnormal vaginal bleeding. Pathologist should be informed of therapy when submitting endometrial tissue for histologic evaluation.
Pregnancy
Pregnancy Considerations
Use is contraindicated during pregnancy. First trimester exposure of progestins may cause genital abnormalities including hypospadias in male infants and mild virilization of external female genitalia. Changes in external genitalia have been reported in female infants exposed to norethindrone acetate (Fine 1963). Significant adverse events related to growth and development have not been observed following use of oral progestins in contraceptive doses (limited studies).
Norethindrone: Progestin-only contraceptives may be started immediately postpartum (Curtis 2016a; Curtis 2016b). A rapid return to fertility occurs when progestin-only contraceptives are discontinued.
Norethindrone acetate: The contraceptive dose of norethindrone acetate is not known. Barrier contraception is recommended to prevent unintended pregnancy (eg, when treating endometriosis) (Kaser 2012).
Patient Education
What is this drug used for?
- It is used to prevent pregnancy.
- It is used to treat uterine bleeding due to hormonal imbalance.
- It is used to treat endometriosis.
- It is used to treat females who do not have a monthly period cycle.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Nausea
- Vomiting
- Cramps
- Bloating
- Tender breasts
- Trouble sleeping
- Acne
- Weight gain
- Facial skin discoloration
- Menstrual irregularities
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Liver problems like dark urine, feeling tired, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin or eyes.
- Severe cerebrovascular disease like change in strength on one side is greater than the other, trouble speaking or thinking, change in balance, or change in eyesight.
- Blood clots like numbness or weakness on one side of the body; pain, redness, tenderness, warmth, or swelling in the arms or legs; change in color of an arm or leg; chest pain; shortness of breath; fast heartbeat; or coughing up blood.
- Severe headache
- Edema
- Abdominal pain
- Severe dizziness
- Passing out
- Bulging eyes
- Vision changes
- Blindness
- Lump in breast
- Breast pain or soreness
- Nipple discharge
- Vaginal pain, itching, and discharge
- Abnormal vaginal bleeding
- Depression
- Mood changes
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.