Boxed Warning
Cardiovascular disorders (capsule):
Estrogens plus progestin therapy should not be used for the prevention of cardiovascular disorders. The Women's Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of myocardial infarction (MI), stroke, pulmonary embolism (PE), and deep vein thrombosis (DVT) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens 0.625 mg combined with medroxyprogesterone 2.5 mg relative to placebo.
Probable dementia (capsule):
Estrogens plus progestin therapy should not be used for the prevention of dementia. The Women's Health Initiative Memory Study (WHIMS) estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years and older during 4 years of treatment with daily conjugated estrogens 0.625 mg combined with medroxyprogesterone 2.5 mg, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women.
Breast cancer (capsule):
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer.
Risks versus benefits (capsule):
In the absence of comparable data, assume these risks to be similar for other doses of conjugated estrogens and medroxyprogesterone and other combinations and dosage forms of estrogens and progestins. Prescribe progestins with estrogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Capsule, Oral:
Prometrium: 100 mg [contains fd&c red #40, fd&c yellow #10 (quinoline yellow), peanut oil]
Prometrium: 100 mg [DSC] [contains peanut oil]
Prometrium: 200 mg [contains fd&c yellow #10 (quinoline yellow), fd&c yellow #6 (sunset yellow), peanut oil]
Prometrium: 200 mg [DSC] [contains peanut oil]
Generic: 100 mg, 200 mg
Gel, Vaginal:
Crinone: 4% (1.125 g); 8% (1.125 g)
Insert, Vaginal:
Endometrin: 100 mg (21 ea)
Oil, Intramuscular:
Generic: 50 mg/mL (10 mL)
Pharmacology
Mechanism of Action
Natural steroid hormone that induces secretory changes in the endometrium, promotes mammary gland development, relaxes uterine smooth muscle, blocks follicular maturation and ovulation, and maintains pregnancy. When used as part of an ART program in the luteal phase, progesterone supports embryo implantation.
Pharmacokinetics/Pharmacodynamics
Absorption
Vaginal gel: Prolonged; Absorption half-life: Vaginal gel: ~25 to 50 hours
Metabolism
Hepatic
Excretion
Urine (50% to 60% as metabolites); bile, feces (~10%)
Time to Peak
Oral: Within 3 hours; IM: ~8 hours; Intravaginal gel: 3.55 ± 2.48 to 7 ± 2.88 hours; Vaginal insert: 17.3 to 24 hours
Half-Life Elimination
Vaginal gel: 5 to 20 minutes
Protein Binding
~96% to 99%, primarily to albumin (50% to 54%) and cortisol-binding protein (43% to 48%)
Use: Labeled Indications
Oral: Prevention of endometrial hyperplasia in nonhysterectomized, postmenopausal women who are receiving conjugated estrogens; treatment of secondary amenorrhea
IM: Treatment of amenorrhea or abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as submucous fibroids or uterine cancer
Intravaginal gel: Part of assisted reproductive technology (ART) for infertile women with progesterone deficiency; treatment of secondary amenorrhea
Vaginal insert: To support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of ART for infertile women
Use: Off Label
Reduce the risk of recurrent spontaneous preterm birthyes
Based on the American College of Obstetricians and Gynecologists (ACOG) practice bulletin for the prediction and prevention of preterm birth, vaginal progesterone is an effective and recommended treatment option to reduce the risk of recurrent spontaneous preterm birth in appropriately selected women (ie, singleton pregnancy and prior spontaneous preterm singleton birth). Therapy may begin at 16 to 24 weeks (regardless of cervical length) or sooner (ie, <24 weeks) in women who have a cervix <20 mm. Use is not recommended as an intervention for women with multiple gestations ACOG 2012. Of note, in women aged 18 to 39 years with >3 prior unexplained recurrent miscarriages or nonconsecutive losses of pregnancy in the first trimester, the use of vaginal progesterone did not result in a significantly higher rate of live births when administered from a time soon after a positive urinary pregnancy test (no later than 6 weeks gestation) through 12 weeks of gestation Coomarasamy 2015.
Contraindications
Oral: Hypersensitivity to progesterone or any component of the formulation, including peanuts/peanut oil; undiagnosed abnormal genital bleeding; breast cancer (known, suspected, or history of); active deep vein thrombosis, pulmonary embolism, or history of these conditions; active or history of arterial thromboembolic disease (eg, stroke, MI); hepatic impairment or disease; pregnancy.
IM: Hypersensitivity to progesterone or any component of the formulation, including sesame oil/seeds; active or history of thrombophlebitis, thromboembolic disorders, or cerebral apoplexy; undiagnosed vaginal bleeding; hepatic impairment or disease; known or suspected malignancy of the breast or genital organs; missed abortion.
Intravaginal gel: Hypersensitivity to progesterone or any component of the formulation, including palm oil; undiagnosed vaginal bleeding; hepatic impairment or disease; known or suspected malignancy of the breast or genital organs; missed abortion; active thrombophlebitis or thromboembolic disorders, or a history of hormone-associated thrombophlebitis or thromboembolic disorders.
Vaginal insert: Hypersensitivity to progesterone or any component of the formulation; undiagnosed vaginal bleeding; known missed abortion or ectopic pregnancy; hepatic disease; known or suspected malignancy of the breast or genital organs; active or history of arterial or venous thromboembolism or severe thrombophlebitis.
Canadian labeling: Additional contraindications (not in the US labeling):
Capsules: Hypersensitivity to soya; endometrial hyperplasia; classical migraine; partial or complete loss of vision due to ophthalmic vascular disease
Intravaginal gel: Acute porphyria, cerebral apoplexy
Vaginal insert/tablet: Porphyria; undiagnosed vaginal bleeding
Documentation of allergenic cross-reactivity for progestins is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Dosage and Administration
Dosing: Adult
Females:
Amenorrhea: IM: 5 to 10 mg/day for 6 to 8 consecutive days.
Amenorrhea, secondary:
Intravaginal gel: 45 mg (4% gel) every other day for up to a total of 6 doses; if response is inadequate, may increase to 90 mg (8% gel) at same schedule.
Oral: 400 mg once daily at bedtime for 10 days.
ART in patients who require progesterone supplementation:
Intravaginal gel: 90 mg (8% gel) once daily. If pregnancy occurs, may continue treatment for up to 10 to 12 weeks.
Intravaginal insert: 100 mg 2 to 3 times daily starting the day after oocyte retrieval and continuing for up to 10 weeks.
ART in patients with partial or complete ovarian failure:
Intravaginal gel: 90 mg (8% gel) twice daily. If pregnancy occurs, continue treatment for up to 10 to 12 weeks.
Endometrial hyperplasia, prevention (in postmenopausal women with a uterus who are receiving conjugated estrogen daily): Oral: 200 mg once daily at bedtime for 12 days sequentially per 28-day cycle. Note: When treating symptoms of menopause, hormone therapy should be evaluated routinely for appropriate dose, duration, and route of administration for each individual patient based on treatment goals, risk factors, and overall health (NAMS 2017). Combined estrogen/progestin therapy is indicated for postmenopausal persons with a uterus to decrease the risk of endometrial cancer. Individuals who have had a hysterectomy generally do not need a progestin; however, one may be needed if there is a history of endometriosis. Adjust dose based on patient response.
Uterine bleeding (functional): IM: 5 to 10 mg/day for 6 doses.
Prevention of spontaneous preterm delivery (singleton pregnancy and prior preterm birth or short cervix) (off-label use): Intravaginal gel: 90 mg (8% gel) once daily (Hassan 2011; O'Brien 2009). Treatment initiation is recommended before or at gestational week 24 (ACOG 2012).
Dosing: Geriatric
Refer to adult dosing.
Administration
IM: Administer deep IM only. May cause injection-site irritation.
Intravaginal:
Vaginal gel: A small amount of gel will remain in the applicator following insertion. Administer into the vagina directly from sealed applicator. Remove applicator from wrapper; holding applicator on each side, push plunger into applicator until it snaps into place; twist off cap counterclockwise; gently insert into vagina and push plunger.
Vaginal insert: Insert tablet in vagina using disposable applicator provided.
Oral: Administer at bedtime. For patients who experience difficulty swallowing the capsules, taking with a full glass of water in the standing position may be beneficial.
Storage
Injection, intravaginal gel: Store at 20°C to 25°C (68°F to 77°F).
Capsule, vaginal insert: Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). Protect capsules from excessive moisture.
Progesterone Images
Drug Interactions
Anticoagulants: Progestins may diminish the therapeutic effect of Anticoagulants. More specifically, the potential prothrombotic effects of some progestins and progestin-estrogen combinations may counteract anticoagulant effects. Management: Carefully weigh the prospective benefits of progestins against the potential increased risk of procoagulant effects and thromboembolism. Use is considered contraindicated under some circumstances. Refer to related guidelines for specific recommendations. Consider therapy modification
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antifungal Agents (Vaginal): May diminish the therapeutic effect of Progesterone. Avoid combination
Bosentan: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
C1 inhibitors: Progestins may enhance the thrombogenic effect of C1 inhibitors. Monitor therapy
CYP2C19 Inducers (Moderate): May decrease the serum concentration of CYP2C19 Substrates (High risk with Inducers). Monitor therapy
CYP2C19 Inducers (Strong): May increase the metabolism of CYP2C19 Substrates (High risk with Inducers). Management: Consider an alternative for one of the interacting drugs. Some combinations may be specifically contraindicated. Consult appropriate manufacturer labeling. Consider therapy modification
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May increase the metabolism of CYP3A4 Substrates (High risk with Inducers). Management: Consider an alternative for one of the interacting drugs. Some combinations may be specifically contraindicated. Consult appropriate manufacturer labeling. Consider therapy modification
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Dabrafenib: May decrease the serum concentration of CYP2C19 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP2C19 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Enzalutamide: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Concurrent use of enzalutamide with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP3A4 substrate should be performed with caution and close monitoring. Consider therapy modification
Enzalutamide: May decrease the serum concentration of CYP2C19 Substrates (High risk with Inducers). Conversely, concentrations of active metabolites may be increased for those drugs activated by CYP2C19. Management: Concurrent use of enzalutamide with CYP2C19 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP2C19 substrate should be performed with caution and close monitoring. Consider therapy modification
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Herbs (Progestogenic Properties) (eg, Bloodroot, Yucca): May enhance the adverse/toxic effect of Progestins. Monitor therapy
Ivosidenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
Mitotane: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Doses of CYP3A4 substrates may need to be adjusted substantially when used in patients being treated with mitotane. Consider therapy modification
Pomalidomide: Progestins may enhance the thrombogenic effect of Pomalidomide. Management: Canadian pomalidomide labeling recommends caution with use of hormone replacement therapy and states that hormonal contraceptives are not recommended. US pomalidomide labeling does not contain these specific recommendations. Consider therapy modification
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Sincalide: Drugs that Affect Gallbladder Function may diminish the therapeutic effect of Sincalide. Management: Consider discontinuing drugs that may affect gallbladder motility prior to the use of sincalide to stimulate gallbladder contraction. Consider therapy modification
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Ulipristal: Progestins may diminish the therapeutic effect of Ulipristal. Ulipristal may diminish the therapeutic effect of Progestins. Management: Ulipristal for uterine fibroids (Canadian indication): avoid progestins within 12 days of stopping ulipristal; as emergency contraceptive (U.S. indication): avoid progestins within 5 days of stopping ulipristal. Avoid combination
Test Interactions
Increased sulfobromophthalein retention and other hepatic function tests; coagulation tests (increase in prothrombin factors VII, VIII, IX, and X); altered metyrapone test; altered pregnanediol determinations; thyroid function (increase in PBI and butanol extractable protein bound iodine, and decrease in T uptake values).
Adverse Reactions
Intramuscular injection: Frequency not defined:
Cardiovascular: Edema
Central nervous system: Depression, drowsiness, insomnia
Dermatologic: Acne vulgaris, allergic skin rash, alopecia, hirsutism, pruritus, skin rash, urticaria
Endocrine & metabolic: Amenorrhea, change in menstrual flow, galactorrhea not associated with childbirth, weight gain, weight loss
Gastrointestinal: Nausea
Genitourinary: Breakthrough bleeding, breast tenderness, cervical erosion, change in cervical secretions, spotting
Hepatic: Cholestatic jaundice
Hypersensitivity: Anaphylactoid shock
Local: Erythema at injection site, irritation at injection site, pain at injection site
Miscellaneous: Fever
Oral (percentages reported when used in combination with or cycled with conjugated estrogens):
>10%:
Central nervous system: Headache (16% to 31%), dizziness (15% to 24%), depression (19%)
Endocrine & metabolic: Breast tenderness (27%), mastalgia (6% to 16%)
Gastrointestinal: Abdominal pain (20%), bloating (12%)
Genitourinary: Urinary tract abnormality (11%)
Infection: Viral infection (12%)
Neuromuscular & skeletal: Musculoskeletal pain (12%)
1% to 10%:
Cardiovascular: Chest pain (7%)
Central nervous system: Anxiety (8%), fatigue (8%), irritability (8%)
Gastrointestinal: Nausea and vomiting (≤8%), diarrhea (7% to 8%), constipation (3% to <5%), cholecystectomy (2% to <5%)
Genitourinary: Vaginal discharge (10%)
Hematologic & oncologic: Malignant neoplasm of breast (2% to <5%)
Respiratory: Cough (8%)
Frequency not defined:
Cardiovascular: Cerebrovascular accident, deep vein thrombosis, myocardial infarction, pulmonary embolism
Central nervous system: Dementia
<1%, postmarketing and/or case reports: Abnormal gait, abnormal uterine bleeding, acute pancreatitis, aggressive behavior, alopecia, anaphylaxis, arthralgia, asthma, blurred vision, choking sensation, cholestasis, cholestatic hepatitis, circulatory shock, cleft lip, cleft palate, confusion, congenital heart disease, depersonalization, diplopia, disorientation, drowsiness, dysarthria, dysphagia, dyspnea, endometrial carcinoma, facial edema, feeling abnormal, heavy menstrual bleeding, hepatic failure, hepatic necrosis, hepatitis, hypersensitivity reaction, hypertension, hypospadias, hypotension, impaired consciousness, increased liver enzymes, increased serum glucose, intoxicated feeling, jaundice, loss of consciousness, menstrual disease, muscle cramps, ovarian cyst, paresthesia, pruritus, sedation, seizure, slurred speech, spontaneous abortion, stupor, suicidal ideation, syncope, tachycardia, tinnitus, tongue edema, transient ischemic attacks, urticaria, vertigo, visual disturbance, weight gain, weight loss
Vaginal gel (percentages reported with Assisted Reproductive Technology):
>10%:
Central nervous system: Drowsiness (27%), headache (13% to 17%), nervousness (16%), depression (11%)
Gastrointestinal: Constipation (27%), nausea (7% to 22%), muscle cramps (15%), abdominal pain (12%)
Genitourinary: Breast hypertrophy (40%), perineal pain (17%), mastalgia (13%), nocturia (13%)
1% to 10%:
Central nervous system: Pain (8%), dizziness (5%)
Dermatologic: Genital pruritus (5%)
Endocrine & metabolic: Decreased libido (10%)
Gastrointestinal: Diarrhea (8%), bloating (7%), vomiting (5%)
Genitourinary: Vaginal discharge (7%), dyspareunia (6%), genital candidiasis (5%)
Neuromuscular & skeletal: Arthralgia (8%)
Vaginal insert (percentages reported with Assisted Reproductive Technology):
>10%: Gastrointestinal: Abdominal pain (12%)
1% to 10%:
Central nervous system: Headache (3% to 4%), fatigue (2% to 3%)
Endocrine & metabolic: Ovarian hyperstimulation syndrome (7%)
Gastrointestinal: Nausea (7% to 8%), abdominal distention (4%), constipation (2% to 3%), vomiting (2% to 3%)
Genitourinary: Uterine spasm (3% to 4%), vaginal hemorrhage (3%), urinary tract infection (1% to 2%)
<1%, postmarketing, and/or case reports: Peripheral edema, urticaria, vaginal discomfort, vulvovaginal burning, vulvovaginal irritation, vulvovaginal pruritus
Warnings/Precautions
Concerns related to adverse effects:
- Breast cancer: [US Boxed Warning]: Based on data from the Women's Health Initiative (WHI) studies, an increased risk of invasive breast cancer was observed in postmenopausal women using conjugated estrogens (CE) in combination with medroxyprogesterone acetate (MPA). Observational studies noted this risk declines once therapy is discontinued. The WHI study did not observe an increased risk of invasive breast cancer in women with a hysterectomy using CE alone. The risk of breast cancer in postmenopausal patients on hormone therapy may depend upon type of estrogen and/or progestin, dose, timing of therapy initiation, duration of therapy, route of administration, and individual patient characteristics (AACE/ACE [Cobin 2017]; NAMS 2017). Hormone therapy may be associated with increased breast density (NAMS 2017); an increase in abnormal mammogram findings requiring further evaluation has been reported with estrogen alone or in combination with progestin therapy.
- CNS depression: May cause CNS depression, which may impair physical or mental abilities; patients must be cautioned about performing tasks that require mental alertness (eg, operating machinery, driving).
- Dementia: [US Boxed Warning]: Progestin plus estrogens should not be used for the prevention of dementia. In the Women's Health Initiative Memory Study (WHIMS), an increased risk of developing probable dementia was observed in women ≥65 years with daily conjugated estrogens combined with medroxyprogesterone. Because the WHI memory studies were conducted in women ≥65 years of age, it is unknown if these findings apply to younger postmenopausal women. However, hormone therapy is not recommended at any age to prevent or treat cognitive decline or dementia (AACE [Goodman 2011]; NAMS 2017).
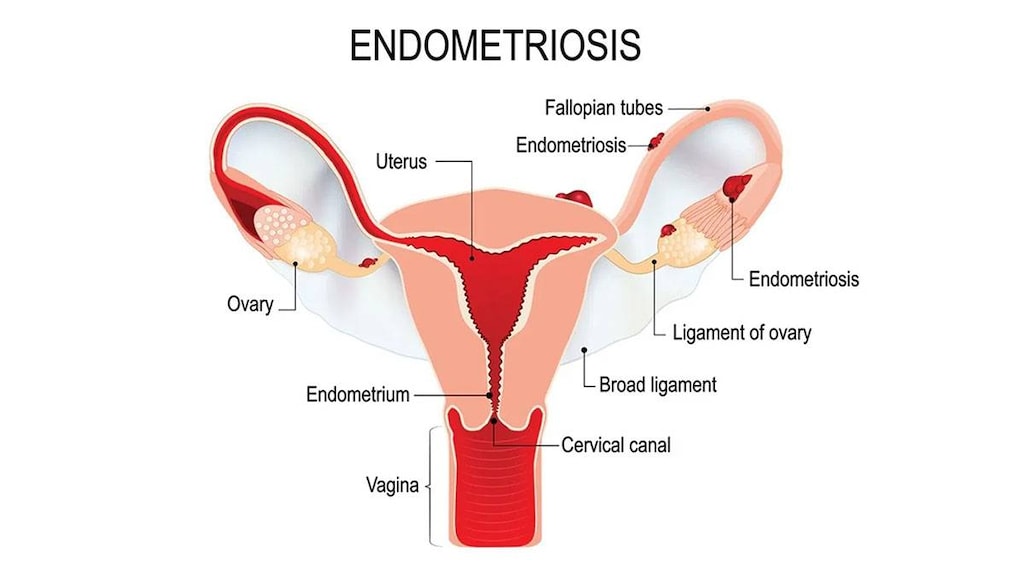
- Endometrial cancer: Progesterone is used to reduce the risk of endometrial hyperplasia in nonhysterectomized postmenopausal women receiving conjugated estrogens. The use of unopposed estrogen in women with an intact uterus is associated with an increased risk of endometrial cancer. The addition of a progestin to estrogen therapy may decrease the risk of endometrial hyperplasia, a precursor to endometrial cancer. Adequate diagnostic measures, including endometrial sampling if indicated, should be performed to rule out malignancy in postmenopausal women with undiagnosed abnormal persistent or recurring vaginal bleeding.
- Endometriosis: Estrogens may exacerbate endometriosis. Malignant transformation of residual endometrial implants has been reported posthysterectomy with unopposed estrogen therapy. Consider adding a progestin in women with residual endometriosis posthysterectomy.
- Eosinophilic pneumonia: Cases of acute eosinophilic pneumonia have been reported with use of progesterone injection in sesame oil; symptoms have included fever, dyspnea with hypoxic respiratory insufficiency, and diffuse pulmonary infiltrates and generally develop 2 to 4 weeks after treatment initiation. Discontinue immediately and undergo prompt medical evaluation if symptoms of eosinophilic pneumonia occur.
- Fluid retention: May cause fluid retention; use with caution in patients with diseases which may be exacerbated by fluid retention, including asthma, cardiac impairment, epilepsy, migraine, and diabetes.
- Ovarian cancer: Available information related to the use of menopausal estrogen or estrogen/progestin therapy and risk of ovarian cancer is inconsistent. If an association is present, the absolute risk is likely rare and may be influenced by duration of therapy (AACE [Goodman 2011]; ES [Stuenkel 2015]; NAMS 2017).
- Retinal vascular thrombosis: Discontinue pending examination in cases of sudden partial or complete vision loss, sudden onset of proptosis, diplopia, or migraine; discontinue permanently if papilledema or retinal vascular lesions are observed on examination.
Disease-related concerns:
- Cardiovascular disease: [US Boxed Warning]: Progestin plus estrogen should not be used for the prevention of cardiovascular disease. In the WHI studies, an increased risk of DVT, PE, stroke, and myocardial infarction was observed in women ≥65 years with daily conjugated estrogens combined with medroxyprogesterone. Additional risk factors include diabetes mellitus, hypercholesterolemia, hypertension, SLE, obesity, tobacco use, and/or history of venous thromboembolism (VTE). Risk factors should be managed appropriately; discontinue use if adverse cardiovascular events occur or are suspected.
- Depression: Use with caution in patients with a history of depression.
- Diabetes: May impair glucose tolerance; use caution in patients with diabetes. Prior to therapy, consider age, cardiovascular and metabolic risk factors in patients previously diagnosed with diabetes (AACE/ACE [Cobin 2017]).
- Hepatic impairment: Use is contraindicated in hepatic impairment or disease.
- Renal impairment: Use with caution in patients with renal impairment; progestins may cause fluid retention.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pediatric: Not for use prior to menarche.
- Surgical patients: Whenever possible, progestins in combination with estrogens should be discontinued at least 4 to 6 weeks prior to elective surgery associated with an increased risk of thromboembolism or during periods of prolonged immobilization.
Dosage form specific issues:
- Benzyl alcohol and derivatives: Some dosage forms may contain benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity ("gasping syndrome") in neonates; the "gasping syndrome" consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol with caution in neonates. See manufacturer's labeling.
- Palm oil: Some products may contain palm oil.
- Peanut oil: Some products may contain peanut oil.
- Sesame oil: Some products may contain sesame oil.
Other warnings/precautions:
- Risks vs benefits: Oral: When used for the relief of menopausal symptoms, the benefit-risk of hormone therapy is most favorable if started in patients who have no contraindications to therapy, are <60 years of age, within 10 years of menopause onset, have a favorable lipid profile, and do not have the factor V Leiden genotype or metabolic syndrome. Risk factors for cardiovascular disease should also be considered when evaluating therapy and route of administration (AACE/ACE [Cobin 2017]; NAMS 2017). [US Boxed Warning]: Progestin with estrogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman. Patients should be reevaluated as clinically appropriate to determine if treatment is still necessary. Available data related to treatment risks are from WHI studies, which evaluated oral conjugated estrogens with medroxyprogesterone relative to placebo in postmenopausal women. Other combinations and dosage forms of estrogens and progestins were not studied. Outcomes reported from clinical trials using conjugated estrogens with medroxyprogesterone should be assumed to be similar for other doses and other dosage forms of estrogens and progestins until comparable data becomes available.
Monitoring Parameters
Menopause: Prior to combination hormonal therapy, baseline risk for breast cancer and CVD. During therapy, age appropriate breast and pelvic exams; blood pressure; unscheduled bleeding lasting >6 months for endometrial pathology (sooner in patients who are obese, diabetic, or have a history of endometrial cancer); serum triglycerides (2 weeks after starting therapy in patients with baseline level >200 mg/dL); TSH (6 to 12 weeks after starting oral therapy in patients taking thyroid replacement); efficacy beginning 1 to 3 months after starting therapy, then every 6 to 12 months as appropriate. Duration of treatment should be evaluated at least annually (ES [Stuenkel 2015]).
Pregnancy
Pregnancy Risk Factor
B (oral)
Pregnancy Considerations
The oral capsules are contraindicated for use during pregnancy.
Adverse events following maternal use in pregnancy (eg, hypospadias, congenital heart disease, cleft lip/palate) have been noted in postmarketing data; however, a causal relationship has not been clearly established. Use of vaginal progesterone may be considered to decrease the risk of recurrent spontaneous preterm birth in women with a singleton pregnancy and prior spontaneous preterm singleton birth (therapy may begin at 16 to 24 weeks, regardless of cervical length). It may also be used to prevent spontaneous preterm birth in women with a singleton pregnancy who have a cervix <20 mm before or at 24 weeks' gestation. Use is not recommended as an intervention for women with multiple gestations (ACOG 2012). The vaginal gel and insert are indicated for use in ART.
Patient Education
What is this drug used for?
- It is used in in vitro fertilization.
- It is used to lower the chance of endometrial changes in women after change of life who are getting estrogen therapy.
- It is used to treat uterine bleeding due to hormonal imbalance.
- It is used to treat females who do not have a monthly period cycle.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Fatigue
- Trouble sleeping
- Nausea
- Vomiting
- Headache
- Increased hunger
- Constipation
- Acne
- Hair loss
- Hair growth
- Abdominal pain
- Abdominal cramps
- Weight loss
- Bloating
- Diarrhea
- Enlarged breasts
- Tender breasts
- Muscle pain
- Back pain
- Joint pain
- Loss of strength and energy
- Decreased sex drive
- Menstrual changes
- Injection site irritation
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Severe cerebrovascular disease like change in strength on one side is greater than the other, trouble speaking or thinking, change in balance, or vision changes
- Blood clots like numbness or weakness on one side of the body; pain, redness, tenderness, warmth, or swelling in the arms or legs; change in color of an arm or leg; chest pain; shortness of breath; fast heartbeat; or coughing up blood
- Ovarian hyperstimulation syndrome like severe abdominal pain or bloating; severe nausea, vomiting, or diarrhea; excessive weight gain; shortness of breath; or change in amount of urine passed
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin
- Shortness of breath
- Trouble breathing
- Cough
- Excessive weight gain
- Abnormal gait
- Severe dizziness
- Passing out
- Bulging eyes
- Vision changes
- Blindness
- Contact lens intolerance
- Lump in breast
- Breast soreness or pain
- Nipple discharge
- Flushing
- Fast heartbeat
- Abnormal heartbeat
- Vaginal pain, itching, and discharge
- Abnormal vaginal bleeding
- Painful urination
- Passing a lot of urine
- Depression
- Mood changes
- Trouble with memory
- Seizures
- Swelling of arms or legs
- Swelling
- Pelvic pain
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.